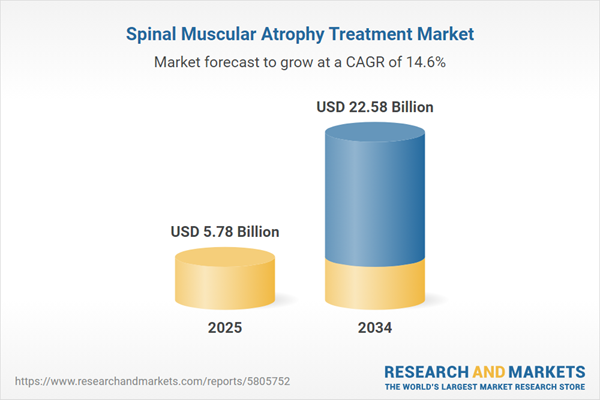
Spinal Muscular Atrophy Treatment Market Overview
Spinal muscular atrophy (SMA) treatment focuses on improving motor function, slowing disease progression, and enhancing quality of life. Therapies include gene replacement therapy, such as onasemnogene abeparvovec, which targets the genetic cause of SMA. Antisense oligonucleotide drugs, like nusinersen, enhance SMN protein production, improving nerve function. Risdiplam, an oral therapy, increases SMN protein levels systemically. Supportive care, including physiotherapy, respiratory support, and nutritional management, is crucial for patient well-being. Advances in gene therapy and personalised medicine continue to transform SMA treatment, offering improved prognosis and mobility for affected individuals.Spinal Muscular Atrophy Treatment Market Growth Drivers
Increasing Adoption of Innovative Therapies to Drive Market Growth
The rising prevalence of spinal muscular atrophy and the increasing demand for advanced therapies are significant market drivers. Additionally, ongoing innovations in muscle-targeted therapies and enhanced treatment regimens are expected to fuel market growth. For instance, in October 2024, Scholar Rock announced promising results from the Phase 3 SAPPHIRE clinical trial evaluating apitegromab, an investigational muscle-targeted treatment for SMA. The therapy demonstrated a clinically significant improvement in motor function, offering a potential treatment option for SMA patients currently on standard care therapies. These positive results are poised to enhance treatment outcomes and drive market expansion in the forecast period.Regulatory Advancements to Meet Rising Spinal Muscular Atrophy Treatment Market Demands
The growing emphasis on early diagnosis and treatment of SMA alongside evolving therapeutic solutions is propelling the treatment market. For instance, In January 2025, Biogen Inc. revealed that both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) accepted applications for a higher dose regimen of nusinersen, which will offer a more rapid loading dose and extended maintenance dosing. This improved dosing regimen promises to optimise treatment efficacy, addressing unmet needs in SMA care and further expanding the therapeutic market. This development is anticipated to boost market growth in the coming years.Spinal Muscular Atrophy Treatment Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Gene Therapy Advancements Driving Market Growth
The market is experiencing substantial growth driven by advancements in gene therapy and biologics. Breakthrough therapies like onasemnogene abeparvovec and nusinersen are transforming spinal muscular atrophy care by targeting the root cause of the disease. Gene therapy, offering long-term benefits, is expected to be a key driver in market expansion. Additionally, ongoing research into personalized medicine and novel drug formulations is further boosting market growth. As new treatments receive regulatory approvals and are widely adopted, the market will continue to evolve, enhancing patient outcomes and providing hope for those affected by spinal muscular atrophy.Technological Advancements Driving the Spinal Muscular Atrophy Treatment Market Value
Technological innovations are shaping the development of spinal muscular atrophy treatments, particularly in gene therapy and RNA-based therapies. With advancements in genome editing techniques and improved drug delivery systems, targeted therapies are becoming more effective in managing SMA. Companies are now focusing on developing treatments that provide long-term disease-modifying effects, addressing the underlying genetic causes of spinal muscular atrophy. These breakthroughs not only improve treatment outcomes but also contribute to the market's progression. Research into new drug formulations, including oral therapies, offers hope for better access and patient compliance, driving the expansion of the treatment market in the future.Increased Funding and Investments Fuelling the Spinal Muscular Atrophy Treatment Market Growth
Increased funding and investments in spinal muscular atrophy research and treatment development are significantly enhancing the market's value. Pharmaceutical companies and biotech firms are investing in innovative therapies and clinical trials aimed at improving SMA care. Government initiatives, along with private sector partnerships, have helped accelerate the development of gene therapies, which are expected to lead to higher treatment adoption rates. As these treatments gain approval and demonstrate long-term effectiveness, the market value is expected to rise substantially. With a growing number of treatments entering the market, spinal muscular atrophy therapy will continue to be a highly lucrative and evolving sector.Rising Awareness and Early Diagnosis to Promote Spinal Muscular Atrophy Treatment Market Demand
Rising awareness of spinal muscular atrophy, along with advances in early diagnosis, is contributing to the expansion of the treatment market. Early detection, through genetic screening and diagnostic advancements, allows for quicker interventions, improving patient outcomes. As awareness grows about the benefits of early treatment and the availability of novel therapies, there is a greater focus on patient advocacy, which in turn drives market growth. Additionally, improved access to healthcare resources and diagnostic tools is expected to lead to more timely diagnoses and better management, supporting the continued expansion of the SMA treatment market worldwide.Spinal Muscular Atrophy Treatment Market Segmentation
Spinal Muscular Atrophy Treatment Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segmentsMarket Breakup by Type
- Type 1
- Type 2
- Type 3
- Type 4
Market Breakup by Procedure
- Gene Replacement Therapy
- Drug Therapy
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
Market Breakup by End User
- Hospitals
- Clinics
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Spinal Muscular Atrophy Treatment Market Share
Type 1 to Lead the Segmentation by Type
Type 1 spinal muscular atrophy (SMA) is the most severe and prevalent form, accounting for a significant portion of the spinal muscular atrophy patient population. Due to the urgent need for effective treatments, this segment is expected to hold the largest market share in the forecast period. Advances in gene therapies, such as Zolgensma, which specifically targets Type 1 SMA, are driving market growth. As these therapies offer life-changing benefits, the demand for treatments for Type 1 SMA will continue to increase. The success of Type 1 treatment options sets the tone for future growth in the treatment market.Gene Replacement Therapy to Dominate Spinal Muscular Atrophy Treatment Market Segmentation by Procedure
Gene replacement therapy is poised to emerge as a leading procedure segment in the treatment market. The success of therapies like Zolgensma has made gene replacement a groundbreaking treatment option. By addressing the root cause of SMA, gene therapy provides long-term, transformative results compared to traditional drug therapies. With ongoing advancements in gene editing techniques and regulatory support, this segment is poised to dominate. The growing acceptance of gene therapies, along with their ability to offer permanent cures, is expected to propel this segment forward, driving significant market expansion in the coming years.
Parenteral Route to Hold a Major Spinal Muscular Atrophy Treatment Market Value by Route of Administration
The parenteral route of administration, particularly intravenous (IV) injection or infusion, is amongst the commonly used methods for delivering spinal muscular atrophy treatments. With gene therapies like Zolgensma requiring intravenous infusion, this route of administration is likely to hold the largest market share in the forecast period. Parenteral delivery ensures optimal bioavailability, especially for biologic therapies that need to bypass the digestive system for effective absorption. The trend towards IV-based gene therapies, with their ability to provide targeted and long-lasting effects, will continue to drive the market for parenteral SMA treatments.
Hospitals to Lead the Spinal Muscular Atrophy Treatment Market by End User
Hospitals are expected to lead the end-user segment for spinal muscular atrophy treatments due to the critical nature of SMA care and the specialised treatments required. These institutions are equipped with the necessary infrastructure, medical professionals, and facilities to administer complex therapies such as gene replacement and drug therapies. As SMA treatments become more advanced and require specialised monitoring and administration, hospitals are expected to maintain dominance in the market. With an increasing number of healthcare institutions adopting cutting-edge SMA therapies, hospitals are poised to drive the highest demand and expansion of SMA treatment solutions.Spinal Muscular Atrophy Treatment Market Analysis by Region
The United States is likely to hold the largest market share in the market. This is primarily due to its well-established healthcare infrastructure, high awareness of SMA, and significant investment in biotechnology research. Additionally, the FDA's approval of innovative therapies such as gene therapy and RNA-based treatments has created a conducive environment for market growth. Europe, particularly Germany and the United Kingdom is also witnessing strong market expansion due to favourable reimbursement policies and increasing access to advanced therapies. Japan, with its robust healthcare system, is expected to show growth, while emerging markets like India are anticipated to contribute steadily with rising awareness and healthcare improvements.Leading Players in the Spinal Muscular Atrophy Treatment Market
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Biogen Inc
Founded in 1978 and headquartered in Cambridge, Massachusetts, USA, Biogen Inc. is a global biotechnology company known for its leadership in neuroscience. Specialising in treatments for neurological diseases, Biogen's portfolio includes therapies for spinal muscular atrophy (SMA), multiple sclerosis, and Alzheimer's disease. Their innovations in gene therapies and biologics have positioned Biogen as a key player in the biopharmaceutical industry, particularly in rare and complex neurological disorders. The company is also involved in research aimed at advancing treatments in neurology and neurodegenerative diseases.Novartis AG
Established in 1996 and headquartered in Basel, Switzerland, Novartis AG is a leading global healthcare company focusing on patented prescription medicines. Their portfolio covers innovative pharmaceuticals, generics, and biosimilars, particularly in areas like oncology, immunology, ophthalmology, and neuroscience. Novartis has made significant contributions to the treatment of spinal muscular atrophy (SMA) and other rare diseases. The company continues to invest heavily in research and development, aiming to enhance the lives of patients globally through cutting-edge therapies and pioneering scientific advancements.F. Hoffmann-La Roche Ltd
Founded in 1896 and based in Basel, Switzerland, F. Hoffmann-La Roche Ltd is a multinational healthcare leader in pharmaceuticals and diagnostics. Known for its pioneering work in oncology, immunology, and neurology, Roche’s portfolio includes treatments for rare diseases, autoimmune disorders, and neurological conditions like SMA. The company’s commitment to personalised healthcare has made it a key player in the global biotechnology space. Roche continues to drive innovation in both pharmaceutical therapies and diagnostic tools, improving outcomes for patients worldwide.Ionis Pharmaceuticals Inc
Founded in 1989 and headquartered in Carlsbad, California, USA, Ionis Pharmaceuticals Inc. is a biotechnology company focused on RNA-targeted therapies. Ionis is a pioneer in the development of antisense oligonucleotide therapies and is known for its role in advancing treatments for rare genetic disorders, including spinal muscular atrophy (SMA). The company’s innovative pipeline includes therapies for a range of conditions, from neurological to cardiovascular diseases. Ionis collaborates with global pharmaceutical companies to expand its reach and impact in treating complex diseases.Other key players in the market include Cytokinetics Inc., PTC Therapeutics, Catalyst Pharmaceuticals, Astellas Pharma Inc., Pfizer Inc., and NMD Pharma A/S.
Key Questions Answered in the Spinal Muscular Atrophy Treatment Market
- What was the spinal muscular atrophy treatment market value in 2024?
- What is the spinal muscular atrophy treatment market forecast outlook for 2025-2034?
- What is market segmentation based on type?
- What is market segmentation based on procedure?
- What is market segmentation based on routes of administration?
- What is market segmentation based on end users?
- What are the major factors aiding the spinal muscular atrophy treatment market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major spinal muscular atrophy treatment market trends?
- Which type will lead the market segment?
- Which procedure will lead the market segment?
- Which routes of administration will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the spinal muscular atrophy treatment market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Biogen Inc.
- Novartis AG
- F. Hoffmann-La Roche Ltd
- Ionis Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 5.78 Billion |
| Forecasted Market Value ( USD | $ 22.58 Billion |
| Compound Annual Growth Rate | 14.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |