Global Nephrostomy Devices Market- Analysis
Nephrostomy devices are essential medical tools used for draining urine from a blocked kidney. These devices are typically employed in cases where there is an obstruction in the urinary tract, often due to kidney stones, tumours, or other urological conditions. The global market for nephrostomy devices is expanding, driven by the increasing prevalence of urological diseases, the growing ageing population, and the rising adoption of minimally invasive surgical procedures. As healthcare providers and patients seek more efficient and less invasive treatment options, the demand for advanced nephrostomy devices is expected to rise significantly.Market Drivers
Rising Prevalence of Urological Disorders: The increasing incidence of urological conditions, such as kidney stones, tumours, and other urinary obstructions, is a major driver of the nephrostomy devices market. As these conditions become more common, the demand for nephrostomy procedures and associated devices continues to grow.Advancements in Medical Technology: Continuous advancements in medical technology are improving the effectiveness and safety of nephrostomy devices. Innovations in device materials, design, and imaging technologies are enhancing patient outcomes, thereby driving market growth.
Growing Ageing Population: The global ageing population is more prone to urological issues, including kidney-related disorders. As the elderly population increases, so does the demand for nephrostomy procedures, contributing to the expansion of the market.
Increasing Adoption of Minimally Invasive Procedures: There is a growing preference for minimally invasive procedures among healthcare providers and patients. Nephrostomy devices, which are integral to these procedures, are seeing increased adoption due to their ability to reduce recovery times, minimise complications, and improve patient comfort.
Favourable Reimbursement Policies: Supportive reimbursement policies in key markets are encouraging the use of nephrostomy devices. These policies reduce the financial burden on patients, making advanced urological treatments more accessible and driving the market forward.
Market Challenges
Risk of Complications: Nephrostomy procedures, while generally safe, carry risks of complications such as infections, bleeding, and device malfunctions. These potential risks can hinder the widespread adoption of nephrostomy devices, particularly among risk-averse patients and healthcare providers.Limited Availability of Skilled Professionals: The successful implementation of nephrostomy procedures requires skilled healthcare professionals. In regions where there is a shortage of trained personnel, the adoption of these devices may be limited, thereby restraining market growth.
Stringent Regulatory Requirements: Navigating the complex regulatory landscape for nephrostomy devices can be challenging for manufacturers. Meeting stringent regulatory standards and obtaining approvals can delay the market entry of new devices, impacting overall market growth.
Patient Awareness and Acceptance: Limited awareness and acceptance of nephrostomy procedures among patients can be a challenge. Educating patients about the benefits and safety of these procedures is essential for increasing adoption rates and market penetration.
Future Opportunities
Development of Cost-Effective Devices: There is an opportunity for manufacturers to develop cost-effective nephrostomy devices that cater to the needs of low- and middle-income regions. Affordable devices can expand market reach and improve accessibility to advanced urological care.Integration with Advanced Imaging Technologies: The integration of nephrostomy devices with advanced imaging technologies, such as ultrasound and MRI, offers significant growth opportunities. These integrated solutions can enhance the accuracy and safety of nephrostomy procedures, driving market adoption.
Collaborations and Partnerships: Collaborations between device manufacturers, research institutions, and healthcare providers can lead to the development of innovative nephrostomy solutions. These partnerships can accelerate product development and market entry, driving growth in the industry.
Focus on Patient-Centric Designs: There is a growing demand for nephrostomy devices that are designed with patient comfort and ease of use in mind. Manufacturers that focus on developing user-friendly devices that enhance patient experience are likely to gain a competitive advantage in the market.
Global Nephrostomy Devices Market Trends
Adoption of Biocompatible Materials: There is an increasing trend towards the use of biocompatible materials in the manufacture of nephrostomy devices. These materials reduce the risk of adverse reactions and improve patient outcomes, making them more appealing to healthcare providers and patients alike.Growth in Home Care Settings: The growing preference for home care settings for post-operative recovery is influencing the demand for portable and easy-to-use nephrostomy devices. This trend is driving innovation in device design, with manufacturers focusing on developing products suitable for home use.
Focus on Infection Control: Infection control is a critical concern in nephrostomy procedures. There is a rising trend towards the development of devices with antimicrobial properties to reduce the risk of infections, thereby improving patient safety and outcomes.
Technological Advancements in Drainage Systems: Technological advancements in nephrostomy drainage systems are enhancing the efficiency and safety of these devices. Innovations such as closed drainage systems and self-flushing mechanisms are gaining popularity, reflecting a shift towards more advanced and reliable solutions.
Increased Use of Disposable Devices: The trend towards disposable nephrostomy devices is gaining traction, particularly in hospitals and clinics. Disposable devices reduce the risk of cross-contamination and simplify the logistics of sterilisation, making them a preferred choice in many healthcare settings.
Global Nephrostomy Devices Market Segmentation
Market Breakup by Product
- Percutaneous Nephrostomy Devices
- Nephrostomy Drainage Bags
- Nephrostomy Kits
- Accessories and Consumables
Market Breakup by Product Material
- Silicone
- Polyurethane
- Others
Market Breakup by Procedure Type
- Diagnostic Nephrostomy
- Therapeutic Nephrostomy
- Emergency Nephrostomy
Market Breakup by End User
- Hospitals and Clinics
- Home Care Settings
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Nephrostomy Devices Market Competitive Landscape
The global nephrostomy devices market features key players such as Boston Scientific Corporation, Cook Medical Inc., Uresil LLC, Teleflex, Inc., Coloplast Corporation, Argon Medical Devices, Inc., Becton, Dickinson, and Company, Olympus Corporation, B. Braun SE, Cardinal Health, Inc. These companies are at the forefront of innovation, focusing on the development of advanced nephrostomy devices that improve patient outcomes and streamline procedures. By leveraging cutting-edge technology and addressing the needs of healthcare providers, these market leaders are driving growth and setting new standards in the nephrostomy devices industry.Key Questions Answered in the Report
- What are the primary factors driving the demand for nephrostomy devices in the healthcare industry?
- What challenges do manufacturers face in the nephrostomy devices market, and how are they addressing them?
- How are advancements in medical technology influencing the development of nephrostomy devices?
- What are the emerging trends in the nephrostomy devices market that are expected to shape its future?
- How does patient preference for minimally invasive procedures impact the demand for nephrostomy devices?
- In what ways are regulatory policies affecting the adoption and innovation of nephrostomy devices globally?
- What role do nephrostomy drainage bags play in the overall market, and how is their demand evolving?
- How are silicone and polyurethane materials contributing to the performance of nephrostomy devices?
- Why is the Asia Pacific region expected to see significant growth in the nephrostomy devices market?
- How are hospitals and clinics driving the demand for nephrostomy devices compared to home care settings?
- What competitive strategies are key players in the nephrostomy devices market employing to strengthen their market position?
Key Benefits for Stakeholders
The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the global nephrostomy devices market from 2017-2032.- The research report provides the latest information on the market drivers, challenges, and opportunities in the global nephrostomy devices market.
- The study maps the leading, as well as the fastest-growing, regional markets, enabling stakeholders to identify key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders analyse the level of competition within the global nephrostomy devices industry and its attractiveness.
- The competitive landscape section allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Boston Scientific Corporation
- Cook Medical Inc.
- Uresil LLC
- Teleflex, Inc.
- Coloplast Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | October 2024 |
| Forecast Period | 2024 - 2032 |
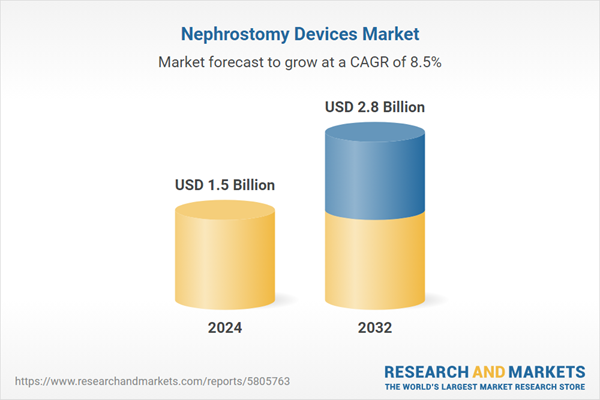
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 2.8 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |