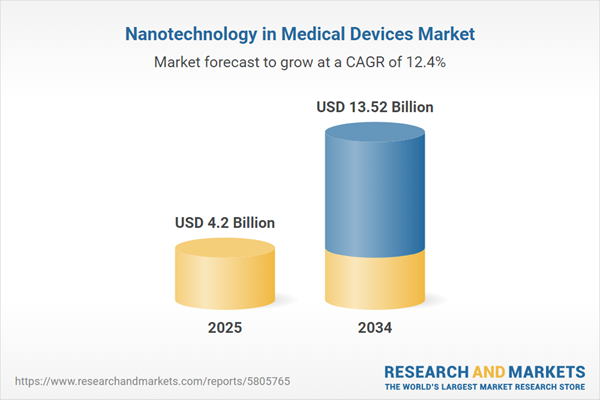
Nanotechnology in Medical Devices Market Overview
Nanotechnology in medical devices enhances diagnostics, treatment, and patient care by utilising nanoscale materials and structures. It improves drug delivery, enabling targeted therapy with reduced side effects. Nano-coatings enhance implant biocompatibility, durability, and infection resistance. Nanosensors provide real-time monitoring of biomarkers, aiding early disease detection. In imaging, nanoparticles improve resolution for accurate diagnosis. Additionally, nanotechnology supports regenerative medicine by promoting tissue engineering and wound healing. With its ability to revolutionise precision medicine and minimally invasive procedures, nanotechnology is driving innovation and efficiency in modern healthcare solutions.Nanotechnology in Medical Devices Market Growth Drivers
AI Integration into Nanotechnology to Enhance Market Value
The increasing demand for non-invasive diagnostics and the integration of AI with nanotechnology are key drivers shaping the market. For instance, in January 2024, Nanowear secured FDA 510(k) clearance for its SimpleSense™ platform, a wearable nanotechnology-enabled device featuring AI-powered Software-as-a-Medical Device (SaMD) for continuous, cuffless blood pressure monitoring. This innovation enhances hypertension diagnostics and expands nanotechnology applications in remote healthcare. The breakthrough is expected to accelerate market expansion by driving the adoption of AI-integrated nano wearables, improving real-time patient monitoring, and supporting personalised healthcare solutions, reinforcing the role of nanotechnology in transforming medical diagnostics during the forecast period.Nanotechnology in Medical Devices Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Nanofluidic Devices Driving Innovation in the Market
The rising demand for precision medicine and advancements in nanoscale manipulation technologies are significantly shaping the nanotechnology in medical devices market. For instance, in December 2024, researchers introduced nanofluidic devices equipped with ultra-narrow channels that precisely manipulate nanoscale objects such as DNA, viruses, and single molecules. By overcoming random molecular motion, these devices enhance diagnostic accuracy, targeted drug delivery, and biosensing applications. This breakthrough is expected to accelerate nanotechnology adoption in medical imaging, personalised medicine, and therapeutic interventions, ultimately driving market growth and expanding the scope of nanotechnology in advanced healthcare solutions in the coming years.Application Across Various Domains to Boost Nanotechnology in Medical Devices Market Demand
Nanotechnology is unlocking new possibilities in personalised medicine and regenerative therapies. Nano-engineered drug delivery systems ensure targeted therapy with minimal side effects, improving patient outcomes. In regenerative medicine, nanomaterials support tissue engineering and wound healing, accelerating recovery. The development of smart nanodevices capable of monitoring biological markers in real time is improving chronic disease management. With the increasing need for advanced treatment solutions, nanotechnology-driven innovations are reshaping the medical devices industry, supporting market development, and expanding applications across various healthcare domains, including neurology, oncology, and orthopaedics.Rising Investments to Accelerate the Nanotechnology in Medical Devices Market Value
Global investments in nanotechnology for medical applications are increasing, driven by government initiatives, private funding, and strategic partnerships. Research institutions and biotechnology firms are focusing on developing advanced nanomaterials for implants, biosensors, and imaging technologies. Companies are securing funding to accelerate nanotech-driven solutions, leading to breakthroughs in non-invasive diagnostics and precision medicine. Regulatory bodies are supporting nanotechnology research, enhancing its integration into mainstream healthcare. As investments grow, the development and commercialisation of nanotechnology-based medical devices are set to expand, positioning the market for significant long-term growth and technological progress.Increasing Adoption of Nanotechnology to Enhance the Nanotechnology in Medical Devices Market Size
The healthcare industry is witnessing a surge in the adoption of nanotechnology-based medical devices due to their superior performance and patient-centric benefits. Nano-enabled devices improve drug efficacy, reduce side effects, and enable early disease detection through enhanced imaging techniques. The rising prevalence of chronic diseases, coupled with increasing demand for minimally invasive procedures, is accelerating the adoption of nanotechnology in healthcare. Additionally, nanotechnology’s role in precision medicine and point-of-care diagnostics is driving its market value, as more healthcare providers and patients embrace these advanced solutions for improved treatment outcomes.Nanotechnology in Medical Devices Market Segmentation
Nanotechnology in Medical Devices Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type of Nanotechnology
- Nanomaterials
- Nanostructures
- Nanocoatings
Market Breakup by Device
- Instruments and Diagnostic Devices
- Therapeutic Devices
- Wound Care Products
Market Breakup by Application
- Drug Delivery Systems
- Diagnostic Devices
- Therapeutic Devices
- Wound Care
- Implants and Prosthetics
Market Breakup by End User
- Hospitals and Clinics
- Diagnostic Centers
- Pharmaceutical Companies
- Research Institutions
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Nanotechnology in Medical Devices Market Share
Nanomaterials to Lead the Segment by Type of Nanotechnology
Nanomaterials are expected to dominate the nanotechnology in medical devices market due to their extensive use in drug delivery, imaging, and diagnostics. As per the analysis by Expert Market Research, the global nanomaterials market is projected to grow at a CAGR of 15.5% between 2025 and 2034. Their ability to enhance biocompatibility, improve therapeutic efficacy, and enable targeted drug release makes them highly valuable. The rising demand for precision medicine, coupled with continuous research in nanomaterial-based medical applications, is further driving their adoption. As advancements in nanotechnology accelerate, nanomaterials are set to play a crucial role in next-generation medical devices, making them a key contributor to market growth during the forecast period.Therapeutic Devices to Dominating the Nanotechnology in Medical Devices Market Segmentation by Device
Therapeutic devices are projected to hold the largest market share due to the increasing use of nanotechnology in cancer treatment, regenerative medicine, and minimally invasive surgeries. The integration of nanoparticles in implantable devices and drug-eluting stents has significantly improved treatment outcomes. Growing investments in nanotechnology-based therapeutics, coupled with the rising prevalence of chronic diseases, are further fuelling demand. As healthcare systems adopt advanced nanotech solutions for improved patient care, therapeutic devices will continue to drive market expansion, solidifying their dominance in the coming years.Drug Delivery Systems to Hold a Substantial Nanotechnology in Medical Devices Market Value Based on Segmentation by Application
Drug delivery systems are anticipated to be the largest application segment, driven by the need for targeted, efficient, and minimally invasive drug administration. Nanotechnology enhances bioavailability, reduces side effects, and ensures controlled release, making it ideal for personalised medicine. The increasing adoption of nanocarriers in oncology, neurology, and infectious disease treatments is propelling demand. As pharmaceutical companies invest in nanotechnology-based drug formulations, this segment will continue to expand, shaping the future of medical treatment and significantly contributing to market growth.
Hospitals and Clinics to Lead the Nanotechnology in Medical Devices Market by End User
Hospitals and clinics are expected to dominate the market as they are the primary adopters of nanotechnology-enabled medical devices for diagnostics, treatment, and patient care. The rising integration of nanotech-based imaging, therapeutic, and wound care solutions in hospitals is enhancing treatment efficiency. Increasing healthcare expenditure, coupled with government initiatives promoting advanced medical technologies, is driving adoption. As hospitals continue to upgrade their medical infrastructure with nanotechnology-driven innovations, this segment is poised for substantial growth, reinforcing its leadership in the market.Nanotechnology in Medical Devices Market Analysis by Region
North America is expected to dominate the market due to strong R&D investments, advanced healthcare infrastructure, and the presence of key industry players. The United States, in particular, is driving market growth with extensive government funding, robust nanotechnology patent filings, and strategic collaborations between biotech firms and academic institutions. The region’s early adoption of nanomedicine for targeted drug delivery, diagnostics, and regenerative medicine further strengthens its leadership.Europe follows closely, with Germany and the UK investing in nanotech-driven medical advancements. Meanwhile, Asia Pacific, led by Japan and China, is expanding its market share through government-backed innovation. Latin America and the Middle East & Africa are gradually progressing, focusing on healthcare modernisation.
Leading Players in the Nanotechnology in Medical Devices Market
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Thermo Fisher Scientific Inc
Founded in 2006 and headquartered in Waltham, Massachusetts, USA, Thermo Fisher Scientific Inc. is a global leader in scientific research and healthcare solutions. The company specialises in laboratory equipment, analytical instruments, reagents, and diagnostic tools, supporting pharmaceutical, biotechnology, and healthcare industries. It offers advanced solutions in molecular diagnostics, bioprocessing, and precision medicine. With a strong commitment to innovation, Thermo Fisher drives advancements in drug development, clinical research, and patient care, strengthening its presence in the global life sciences and medical technology markets.Convatec Group
Established in 1978 and headquartered in Reading, United Kingdom, Convatec Group is a leading medical technology company specialising in wound care, ostomy care, continence care, and infusion devices. The company focuses on developing advanced medical solutions to improve patient quality of life, particularly for those with chronic conditions. Convatec’s innovative products support healthcare professionals in delivering effective treatments, enhancing patient comfort, and reducing healthcare costs. With a global footprint, the company continues to expand its portfolio through research, acquisitions, and strategic partnerships in the medical device sector.3M Company
Founded in 1902 and headquartered in Saint Paul, Minnesota, USA, 3M Company is a multinational conglomerate known for its diverse product portfolio across healthcare, safety, and industrial sectors. Its healthcare division offers advanced wound care solutions, infection prevention products, dental and orthodontic supplies, and medical adhesives. 3M integrates nanotechnology and biocompatible materials into its medical innovations, enhancing patient safety and treatment effectiveness. With a strong emphasis on research and sustainability, the company continues to pioneer next-generation medical technologies and expand its influence in the global healthcare industry.Medtronic plc
Headquartered in Dublin, Ireland, and established in 1949, Medtronic plc is a global leader in medical technology, specialising in cardiovascular, neurological, and surgical solutions. The company’s portfolio includes implantable cardiac devices, insulin pumps, spinal implants, and robotic-assisted surgical systems. Medtronic is at the forefront of minimally invasive procedures and digital health innovations, aiming to improve patient outcomes and healthcare efficiency. With a strong global presence and continuous investment in research and development, Medtronic remains a key player in transforming medical care through advanced therapeutic and diagnostic solutions.Other key players in the market include Straumann Holding AG, PerkinElmer Inc., TUV Rheinland AG, General Electric Company, LivaNova PLC, and Ferro Corporation.
Key Questions Answered in the Nanotechnology in Medical Devices Market
- What was the global nanotechnology in medical devices market value in 2024?
- What is the nanotechnology in medical devices market forecast outlook for 2025-2034?
- What is market segmentation based on types of nanotechnology?
- What is market segmentation based on devices?
- What is market segmentation based on application?
- What is market segmentation based on end users?
- What are the major factors aiding nanotechnology in medical devices market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major nanotechnology in medical devices market trends?
- Which type of nanotechnology will lead the market segment?
- Which device will lead the market segment?
- Which application will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the nanotechnology in medical devices market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific Inc.
- Convatec Group
- 3M Company
- Medtronic plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 4.2 Billion |
| Forecasted Market Value ( USD | $ 13.52 Billion |
| Compound Annual Growth Rate | 12.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |