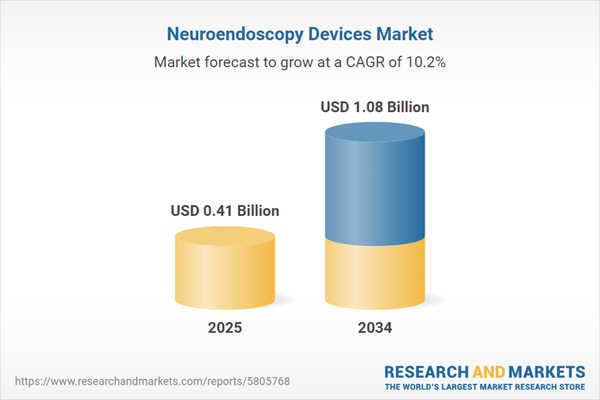
Neuroendoscopy Devices Market Overview
Neuroendoscopy devices are specialised instruments used in minimally invasive neurosurgical procedures to diagnose and treat conditions within the brain, ventricles, and spinal canal. These devices include rigid and flexible neuroendoscopes, equipped with high-resolution cameras and light sources for enhanced visualisation and precision. They enable procedures such as tumour removal, third ventriculostomy, and cyst fenestration, reducing the need for open surgery. Advancements in robotic-assisted systems, high-definition imaging, and fluorescence-guided techniques are improving surgical outcomes, minimising complications, and promoting faster recovery in patients with neurological disorders, hydrocephalus, and brain tumours.Neuroendoscopy Devices Market Growth Drivers
Surge in Strategic Mergers to Drive Growth
The market is expanding due to technological advancements in minimally invasive neurosurgery and the growing integration of digital solutions in operating rooms. For instance, in June 2024, Asensus Surgical, Inc., announced a definitive merger agreement with KARL STORZ Endoscopy-America, Inc., a subsidiary of KARL STORZ SE & Co. KG. This merger will enhance research, innovation, and product development in neuroendoscopy. The combined expertise is expected to accelerate the development of advanced neuroendoscopic systems, strengthening market adoption and technological advancements in the forecast period.Rising Regulatory Approvals to Strengthen Neuroendoscopy Devices Market Size Positively
The rising demand for minimally invasive neurosurgical procedures and growing regulatory approvals for advanced neuroendoscopy systems are key drivers of market growth. For instance, in September 2024, Clearmind Biomedical received U.S. FDA 510(k) clearance for its Neuroblade system, a novel neuroendoscopy device featuring integrated visualisation, illumination, irrigation, suction, coagulation, and powered debridement to enhance surgical precision. This approval is poised to increase market penetration, accelerate adoption in neurosurgical centres, and drive innovation in neuroendoscopic procedures, significantly boosting market expansion in the coming years.Neuroendoscopy Devices Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Rising Brain Tumour Cases to Drive Market Growth
The increasing prevalence of brain tumours is a significant factor boosting the demand for neuroendoscopy devices. According to the National Brain Tumor Society (2023), around 1 million Americans were expected to be diagnosed with brain tumours, with 18,990 deaths anticipated from malignant cases. This growing disease burden is leading to higher adoption of minimally invasive neuroendoscopic procedures, improving patient outcomes and reducing surgical complications. With advancements in imaging and precision-based tools, the market is expected to witness strong growth as healthcare facilities invest in cutting-edge technologies for tumour management.Rising Regulatory Approvals to Enhance Neuroendoscopy Devices Market Size
Regulatory clearances play a crucial role in advancing neuroendoscopy technology and expanding market access. In January 2021, ClearMind Biomedical Inc. received U.S. FDA clearance for its Axonpen neuroendoscope system, enabling safer and more efficient minimally invasive neurosurgeries. Such approvals foster technological innovation, market expansion, and increased hospital adoption. The clearance of new devices enhances surgeons' confidence in neuroendoscopic procedures, leading to higher demand for advanced systems. As more companies seek regulatory approval, the market is expected to experience steady growth, driven by improved accessibility and adoption of neuroendoscopic solutions worldwide.Integration of Artificial Intelligence to Drive Neuroendoscopy Devices Market Growth
Artificial Intelligence (AI) is transforming neuroendoscopy procedures, enhancing real-time surgical precision and decision-making. AI-powered image recognition and augmented reality (AR) integration are improving tumour identification and surgical navigation, reducing complications and operative time. AI-based predictive analytics also assists in preoperative planning, increasing procedure success rates. With major MedTech companies investing in AI-enhanced neuroendoscopy platforms, the market is poised for significant growth. The adoption of AI-driven technologies is expected to increase operational efficiency, optimise patient outcomes, and reduce healthcare costs, contributing to long-term market expansion.Growing Adoption of Single-Use Devices to Boost Neuroendoscopy Devices Market Demand
The rising focus on infection control and cost-effective surgical solutions is driving demand for single-use neuroendoscopes. These devices eliminate the risk of cross-contamination, reduce reprocessing costs, and ensure sterile, high-quality imaging for neurosurgeons. The COVID-19 pandemic heightened the need for disposable medical devices, accelerating market acceptance. With hospitals and ambulatory surgical centres prioritising patient safety, key manufacturers are developing advanced single-use neuroendoscopy systems. As regulatory bodies support safer, disposable alternatives, the market is expected to witness substantial growth, further driving innovation and adoption of cost-effective neuroendoscopic solutions.Neuroendoscopy Devices Market Segmentation
Neuroendoscopy Devices Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Rigid Neuroendoscopes
- Flexible Neuroendoscopes
- Neuroendoscopic Accessories
Market Breakup by Technology
- Conventional Neuroendoscopy
- Advanced Neuroendoscopy
Market Breakup by Endoscopic Technique
- Optical Neuroendoscopy
- Ultrasound Neuroendoscopy
- Laser Neuroendoscopy
Market Breakup by Application
- Brain Tumor Surgery
- Ventriculostomy
- Hydrocephalus Treatment
- Pituitary Tumor Surgery
- Spinal Surgery
- Others
Market Breakup by End User
- Hospitals and Clinics
- Outpatient Facilities
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Neuroendoscopy Devices Market Share
Rigid Neuroendoscopes to Dominate the Segmentation by Product
Rigid neuroendoscopes are expected to hold the largest market share due to their high precision, durability, and effectiveness in neurosurgical procedures. These devices are widely used in brain tumour removal, hydrocephalus treatment, and ventriculostomy due to their superior visualisation and manoeuvrability. The increasing prevalence of neurological disorders and rising demand for minimally invasive surgeries are further driving adoption. Additionally, technological advancements in high-definition optics and camera integration are enhancing procedural outcomes. As hospitals prioritise cost-effective and efficient surgical tools, the demand for rigid neuroendoscopes will continue growing, making them the preferred choice in neuroendoscopic interventions.Advanced Neuroendoscopy to Lead the Market Segmentation by Technology
Advanced neuroendoscopy is poised to dominate the neuroendoscopy devices market segmentation due to continuous innovation in imaging, navigation, and robotic-assisted techniques. The integration of 3D visualisation, fluorescence-guided surgery, and AI-driven assistance is improving surgical accuracy and patient safety. Rising cases of brain tumours and hydrocephalus are further driving adoption. A study reported that in August 2023, hydrocephalus affected approximately 85 per 100,000 people globally, with a higher prevalence of 88 per 100,000 in children. This growing disease burden, coupled with a shift towards minimally invasive neurosurgery, is fuelling demand. Strategic investments in next-generation neuroendoscopic platforms are enhancing procedural efficiency, making advanced neuroendoscopy a key driver of market expansion.Optical Neuroendoscopy to Dominate the Neuroendoscopy Devices Market Value for Segmentation Based on Endoscopic Technique
Optical neuroendoscopy is anticipated to lead the market, driven by its high-definition imaging, real-time visualisation, and enhanced surgical accuracy. This technique is widely used in brain tumour removal, ventriculostomy, and pituitary surgeries, ensuring minimally invasive access to delicate brain structures. Recent advancements in fibre-optic technology and miniaturised endoscopic cameras have further improved procedural efficiency. Growing demand for safer and more effective neurosurgical interventions, coupled with increasing adoption in hospitals and specialised neurosurgery centres, will continue propelling this segment. As precision and safety remain critical, optical neuroendoscopy will dominate the market.
Brain Tumour Surgery to Lead the Neuroendoscopy Devices Market by Application
Brain tumour surgery is projected to hold the largest market share due to rising global incidence rates and growing demand for minimally invasive procedures. As per the analysis by Expert Market Research, the global brain tumor treatment market is expected to grow at a CAGR of about 10.9% during the forecast period of 2025-2034. Neuroendoscopic techniques enable tumour resection with minimal trauma, reducing recovery time and post-operative complications. Advances in endoscopic navigation, fluorescence-guided imaging, and robotic-assisted systems have significantly enhanced surgical outcomes. Additionally, increasing awareness and improved healthcare infrastructure in developing regions are expanding patient access to neuroendoscopic brain tumour procedures. With continuous technological progress and a focus on precision oncology, brain tumour surgery will remain a key driver of the market.Hospitals and Clinics to Dominating the Neuroendoscopy Devices Market Share by End User
Hospitals and clinics are set to dominate due to their high patient volume, availability of advanced neurosurgical equipment, and growing adoption of minimally invasive techniques. Increasing investments in neurosurgery units, coupled with the rising burden of neurological disorders, are boosting demand for neuroendoscopic devices. Furthermore, hospitals are integrating AI-assisted imaging, robotic navigation, and high-definition endoscopic systems to improve surgical precision. As healthcare institutions focus on enhancing neurosurgical capabilities and patient outcomes, the preference for state-of-the-art neuroendoscopic solutions will continue to grow, cementing hospitals and clinics as the primary end users in the market.Neuroendoscopy Devices Market Analysis by Region
North America is expected to hold the largest share due to its advanced neurosurgical infrastructure, high adoption of minimally invasive techniques, and strong presence of key market players. The region’s high incidence of brain tumours and hydrocephalus, along with favourable reimbursement policies, drives demand for neuroendoscopic procedures. Additionally, continuous technological advancements in optical and robotic-assisted neuroendoscopy strengthen market dominance.Europe follows closely, supported by government funding for neurosurgical innovations and rising preference for minimally invasive surgeries. Asia-Pacific is witnessing the fastest growth, driven by expanding healthcare access, medical tourism, and increasing neurological disorder cases. Meanwhile, Latin America and the Middle East & Africa remain emerging markets, benefiting from gradual neurosurgical advancements and healthcare investments.
Leading Players in the Neuroendoscopy Devices Market
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Karl Storz SE & Co. KG
Founded in 1945 and headquartered in Tuttlingen, Germany, Karl Storz SE & Co. KG is a global leader in endoscopic medical technology. The company specialises in rigid and flexible endoscopes, visualisation systems, and surgical instruments across various medical fields, including neurosurgery, ENT, and urology. In the neuroendoscopy market, Karl Storz offers high-definition neuroendoscopes with advanced optics and imaging systems for precise surgical interventions. With a strong focus on innovation, research, and digital integration, Karl Storz continues to develop cutting-edge solutions that enhance minimally invasive neurosurgical procedures worldwide.B. Braun Medical Ltd
Established in 1839, B. Braun Medical Ltd is headquartered in Melsungen, Germany, and is a leading provider of medical and surgical solutions. The company specialises in infusion therapy, surgical instruments, and neuroendoscopic devices, catering to hospitals and healthcare providers globally. In neuroendoscopy, B. Braun offers state-of-the-art rigid and flexible endoscopes designed for minimally invasive neurosurgical procedures. With a commitment to safety, precision, and patient care, the company continuously invests in technological advancements, ensuring improved surgical outcomes and enhanced efficiency in neurosurgical applications across the world.Ackermann Instrumente GmbH
Founded in 1954 and headquartered in Rietheim-Weilheim, Germany, Ackermann Instrumente GmbH specialises in high-quality surgical instruments, endoscopic systems, and medical devices. The company has a strong presence in neuroendoscopy, offering advanced optics, minimally invasive surgical tools, and innovative camera systems. Its precision-engineered neuroendoscopic devices are widely used for brain tumour resection, ventriculostomy, and hydrocephalus treatment. Ackermann Instrumente focuses on continuous product development, ensuring high-performance solutions that enhance neurosurgical precision and safety. With a global reach, the company remains a trusted partner in endoscopic and minimally invasive surgery.Adeor Medical AG
Headquartered in Memmingen, Germany, Adeor Medical AG has been a key player in the medical technology industry for over 40 years. The company specialises in neurosurgical equipment, including high-performance neuroendoscopes, cranial stabilisation systems, and surgical drills. Adeor focuses on precision engineering and innovation, developing cutting-edge neurosurgical instruments that enhance surgical efficiency and patient safety. With a strong emphasis on ergonomics and advanced imaging, Adeor’s solutions support minimally invasive neurosurgery, ensuring optimal surgical outcomes. Its growing global presence and commitment to quality-driven innovation make Adeor a trusted name in neurosurgical advancements.Other key players in the market include Richard Wolf GmbH, Clarus Medical LLC., Tonglu Wanhe Medical Instrument Co., Ltd, Machida Endoscope Co., Ltd, Hangzhou HAWK Optical Electronic Instruments Co., Ltd, and Schindler Endoskopie Technologie GmbH.
Key Questions Answered in the Neuroendoscopy Devices Market
- What was the global neuroendoscopy devices market value in 2024?
- What is the global neuroendoscopy devices market forecast outlook for 2025-2034?
- What is market segmentation based on product?
- What is market breakup based on technology?
- What is market segmentation on the basis of endoscopic technique?
- What is market segmentation based on application?
- What is market segmentation based on end users?
- What are the major factors aiding the global neuroendoscopy devices market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major global neuroendoscopy devices market trends?
- Which product will lead the market segment?
- Which technology will lead the market segment?
- Which endoscopic technique will lead the market segment?
- Which application will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the global neuroendoscopy devices market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Karl Storz SE & Co. KG
- B. Braun Medical Ltd
- Ackermann Instrumente GmbH
- Adeor Medical AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 0.41 Billion |
| Forecasted Market Value ( USD | $ 1.08 Billion |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |