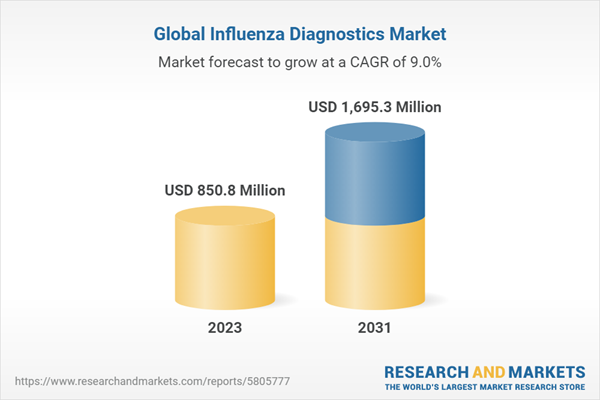
burden of influenza and the need for rapid and accurate diagnosis across the globe. The market size is anticipated to grow at a CAGR of 9% during the forecast period of 2023-2031 to achieve a value of USD 1,695.3 million by 2031.
Influenza Diagnostic: Introduction
Influenza, commonly known as the flu, is a highly contagious respiratory illness caused by influenza viruses. Rapid and accurate diagnosis of influenza is crucial for effective management and control of the disease. Influenza diagnostic tests are designed to identify the presence of the influenza virus in respiratory specimens, aiding in early detection, proper treatment, and prevention of the spread of the virus.Key Trends in the Influenza Diagnostic Market
Some key trends involved in the influenza diagnostic market are as follows:- Growing Demand for Point-of-Care Testing: There is an increasing trend towards point-of-care testing for influenza, which allows for rapid and convenient diagnosis at the patient's location. Point-of-care tests provide quick results, enabling healthcare providers to make timely treatment decisions and take appropriate infection control measures. The demand for user-friendly, portable, and reliable point-of-care testing devices is on the rise
- Technological Advancements: The field of influenza diagnostics is witnessing technological advancements that enhance the accuracy, sensitivity, and speed of diagnostic tests. Molecular diagnostic techniques such as Polymerase Chain Reaction (PCR) and Next-Generation Sequencing (NGS) are becoming more widely used, offering improved sensitivity and the ability to detect multiple strains of the influenza virus. Additionally, the development of rapid diagnostic tests utilizing advanced immunochromatographic and immunoassay techniques is contributing to faster and more efficient diagnosis
- Shift towards Multiplex Testing: Multiplex testing, which allows for the simultaneous detection of multiple respiratory pathogens including influenza, is gaining prominence in the influenza diagnostic market. This approach offers the advantage of identifying co-infections and differentiating between different respiratory viruses, enabling more targeted and accurate treatment decisions. Multiplex testing can help in reducing healthcare costs, optimizing antimicrobial usage, and improving patient outcomes
Influenza Diagnostic Market Segmentations
Market Breakup by Test Type
Traditional Diagnostic Test
- Rapid Influenza Diagnostic Test (RIDT)
- Viral Culture
- Direct Fluorescent Antibody (DFA) Test
- Serological Assay
Molecular Diagnostic Assay
- Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
- Nucleic Acid Sequence-Based Amplification (NASBA) Test
- Loop-Mediated Isothermal Amplification-Based Assay (LAMP)
- Simple Amplification-Based Assay (SAMBA)
- Others
Market by Product
- Test Kits
- Instruments
Market by End User
- Hospitals
- Diagnostic Laboratories
- Research Laboratory
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Influenza Diagnostic Market Scenario
The global influenza diagnostic market plays a critical role in the early detection and management of influenza outbreaks. Influenza, commonly known as the flu, is a contagious respiratory illness caused by influenza viruses. Rapid and accurate diagnosis is crucial to initiate timely treatment, implement appropriate infection control measures, and prevent the spread of the virus.The market for influenza diagnostics encompasses a wide range of products and technologies, including rapid antigen tests, molecular tests, and immunoassays. These diagnostic tools are used in various healthcare settings such as hospitals, clinics, laboratories, and point-of-care facilities.
The market is driven by several factors, including the global burden of influenza, increasing awareness about the importance of early diagnosis, and the development of advanced diagnostic technologies. Additionally, government initiatives and programs aimed at influenza surveillance and control are also contributing to the market growth.
In conclusion, the global influenza diagnostic market is experiencing steady growth due to the increasing burden of influenza and the need for rapid and accurate diagnosis. Advancements in diagnostic technologies, government initiatives, and growing awareness are driving market expansion. As the global healthcare sector continues to prioritize the prevention and control of infectious diseases, the demand for effective influenza diagnostic tools is expected to rise, presenting opportunities for market players to develop innovative solutions and contribute to the fight against influenza outbreaks.
Influenza Diagnostic Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Abbott Laboratories
- Becton, Dickinson and Company
- Coris BioConcept
- DiaSorin SpA
- F. Hoffmann-La Roche Ltd
- Luminex Corporation
- Meridian Bioscience Inc
- Quidel Corporation
- Sekisui Diagnostics
- Thermo Fisher Scientific Inc
- Hologic, Inc.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Becton
- Dickinson and Company
- Coris BioConcept
- DiaSorin SpA
- F. Hoffmann-La Roche Ltd.
- Luminex Corporation
- Meridian Bioscience Inc.
- Quidel Corporation
- Sekisui Diagnostics
- Thermo Fisher Scientific Inc.
- Hologic Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 850.8 Million |
| Forecasted Market Value ( USD | $ 1695.3 Million |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |