Introduction
Autogenous vaccines, also known as autovaccines or autogenous bacterins, are a type of customized vaccine developed specifically for individual animals or groups of animals within a specific farm or herd. Unlike commercial vaccines that are produced on a large scale for general use, autogenous vaccines are tailored to target specific pathogens present on a particular farm or in a specific population of animals. These vaccines are typically developed using the pathogens isolated from the affected animals and are designed to stimulate the animal's immune system to produce a specific immune response against those pathogens.Autogenous vaccines play a crucial role in the management and control of infectious diseases in livestock, poultry, and aquaculture. They are particularly useful in situations where commercial vaccines may not provide adequate protection due to the unique strain or variant of the pathogen present in the specific farm or herd. Autogenous vaccines help to reduce the incidence and severity of infectious diseases, improve animal health and welfare, and minimize economic losses associated with disease outbreaks.
Key Trends in the Autogenous Vaccines Market
Some key trends involved in the autogenous vaccines market are as follows:- Increasing Demand for Customized Vaccines: The demand for autogenous vaccines is growing as livestock producers, poultry farmers, and aquaculture operators recognize the need for customized solutions to address specific disease challenges on their farms. This trend is driven by the desire for more targeted and effective disease control measures and the increasing awareness of the limitations of commercial vaccines
- Advances in Biotechnology: Technological advancements in biotechnology, including genomics and proteomics, have facilitated the development of autogenous vaccines. These tools enable researchers to identify and characterize specific strains or variants of pathogens, allowing for the design and production of autogenous vaccines that are more tailored and effective
- Regulatory Support and Guidelines: Regulatory bodies and organizations governing animal health and production are increasingly recognizing the importance of autogenous vaccines and have established guidelines and regulations for their development and use. This trend provides a more streamlined and standardized approach to the production and application of autogenous vaccines, ensuring their safety, efficacy, and quality
- Collaboration and Partnerships: Collaborations between research institutions, vaccine manufacturers, and livestock producers are becoming more common in the development and deployment of autogenous vaccines. These partnerships facilitate the sharing of knowledge, resources, and expertise, leading to the development of more effective and accessible autogenous vaccines
Autogenous Vaccines Market Segmentations
Market Breakup by Strain Type
- Bacterial Strain
- Virus Strain
Market Breakup by End User
- Veterinary Clinics
- Veterinary Hospitals
- Veterinary Research Institutes
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Autogenous Vaccines Market Scenario
The autogenous vaccine market is a rapidly evolving segment within the animal health industry. Autogenous vaccines are customized solutions developed for specific farms or herds to address the unique pathogen challenges they face. These vaccines are gaining traction as a valuable tool in disease management and prevention, offering tailored protection against pathogens that may not be effectively targeted by commercial vaccines.The market for autogenous vaccines is primarily driven by the increasing need for more targeted and efficient disease control strategies in livestock, poultry, and aquaculture production. Factors such as the rising global demand for animal protein, the increasing incidence of infectious diseases, and the growing emphasis on animal welfare and health are fueling the demand for autogenous vaccines.
The market is witnessing technological advancements in areas such as genomics, proteomics, and bioinformatics, which enable researchers to identify and characterize specific strains or variants of pathogens. This knowledge helps in the development of highly specific autogenous vaccines with enhanced efficacy and safety profiles.
Regulatory bodies and organizations are recognizing the importance of autogenous vaccines and are providing guidelines and regulations to ensure their quality, safety, and efficacy. This regulatory support fosters standardization and increases confidence among producers and veterinarians in the use of autogenous vaccines.
Overall, the autogenous vaccine market presents significant growth opportunities. The increasing demand for customized disease control solutions, technological advancements, regulatory support, collaborations, and the emphasis on preventive healthcare are key drivers shaping the market landscape. As the awareness and adoption of autogenous vaccines continue to grow, the market is expected to expand, offering new avenues for innovation and improved animal health outcomes.
Autogenous Vaccines Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Newport Laboratories, Inc
- Elanco Animal Health Inc
- Cambridge Technologies
- Hygieia Biological Laboratories
- AniCon Labor GmbH
- Boehringer Ingelheim International GmbH
- Ceva Biovac
- Phibro Animal Health Corporation
- Ace Laboratory Services
- Huvepharma, Inc.
Table of Contents
Companies Mentioned
- Newport Laboratories Inc.
- Elanco Animal Health Inc
- Cambridge Technologies
- Hygieia Biological Laboratories
- AniCon Labor GmbH
- Boehringer Ingelheim International GmbH
- Ceva Biovac
- Phibro Animal Health Corporation
- Ace Laboratory Services
- Huvepharma Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
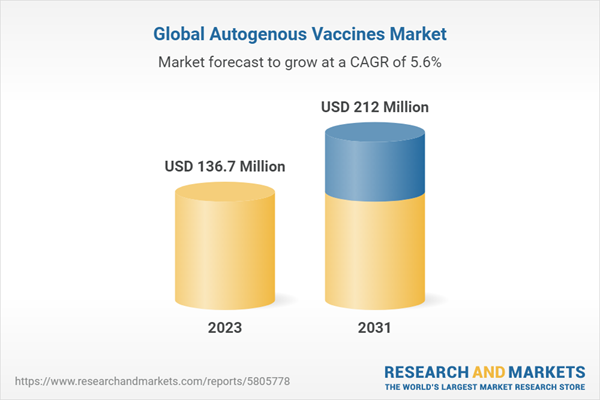
| Estimated Market Value ( USD | $ 136.7 Million |
| Forecasted Market Value ( USD | $ 212 Million |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |