Veterinary pharmacovigilance is the study, assessment, and improvement of the safety of veterinary drugs, focusing on adverse events related to drugs in animals. Also, information on adverse events (AE) resulting from off-label usage is gathered, and the validity of the relevant environmental issues and the withdrawal period are examined.
Pharmacovigilance additionally considers the suspected absence of expected efficacy and any issues with veterinary medicine residues in animal-derived foods where the proper withdrawal period and dose rate have been observed. An adverse event (AE) is any undesirable or unexpected occurrence in animals, individuals, or the environment that occurs after the administration of veterinary medicine, regardless of whether or not it is believed to be related to the product.
An AE can occur at any time during or after treatment with the medication. The independent regulatory agencies have put all approved veterinary medications through a thorough evaluation procedure that examines their quality, safety, and efficacy. The FDA approves safe medications for sale when the advantages to animals exceed any known potential dangers. The product documentation's safety precautions and pertinent usage and disposal instructions may also help to reduce potential hazards or unpleasant outcomes.
As part of the ongoing description and scientific evaluation of the product's safety and effectiveness, there is also a monitoring and assessment process. These pharmacovigilance operations enable the identification of any possible adverse effects that might manifest when drugs are administered to a wider and more varied population of animals or under field conditions An AE known as a Suspected Adverse Reaction (SAR) is when side effects appear in humans or animals following a veterinary treatment.
COVID-19 Impact Analysis
The COVID-19 pandemic promoted an understanding of the significance of pharmacovigilance reporting to safeguard both human and animal health. Additionally, it encouraged end users, like veterinary CROs, manufacturers, etc., to use and switch to electronic reporting systems and software. Although the market continued to encounter obstacles like underreporting of adverse medication events during the pandemic, the increased vigilance because of COVID-19 has aided in the advancement of the market.Market Growth Factors
Increasing zoonotic disease cases and encouraging research
Before the COVID-19 pandemic, estimates showed that zoonosis yearly resulted in 2.7 million fatalities and 2.5 billion cases of human illness. In developing countries as well, zoonoses constitute a serious burden. For example, the International Livestock Research Institute (ILRI) states that zoonotic diseases are a significant problem in low-income countries. For the escalating disease outbreaks in emerging countries to stop causing financial losses, better animal treatment and medications are required. This has sparked renewed interest in veterinary pharmacovigilance, which will hasten the market's growth.Rising requirement for pharmacovigilance reporting to propagate R&D and product launches
Pharmacovigilance aims to identify any developing signal as soon as possible, whether it be an unanticipated or recognized adverse reaction but with an unusual frequency or severity. Then, as necessary, the risk management strategies can be adjusted owing to the post-MA monitoring, including everything from a warning to the packaging leaflet to canceling the marketing authorization. Additionally, the program's efficiency depends on spontaneous declarations, which veterinarians send in most cases. Therefore, the rising demand for pharmacovigilance is boosting the growth of the market.Market Restraining Factors
Lack of efficient reporting framework in many nations
Most people do not report the AEs because of doubts about whether the occurrence they had observed constituted an AE. This is even more obvious in the case of ineffectiveness when most people state that it was difficult to identify or notice ineffectiveness. Additional factors considered critical in preventing people from reporting were that the adverse occurrences weren't serious enough or had already been known, as well as the fact that the reporting process "takes too long." Fear of affecting product availability or "getting the reporter or their client in trouble" also prevents people from reporting. Thus, the lack of efficient infrastructure and framework for reporting AEs may obstruct the expansion of the market.Solution Outlook
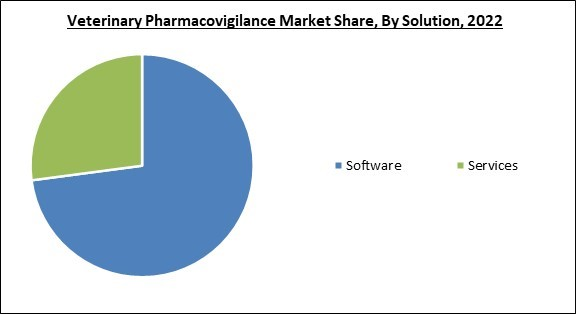
Based on solution, the veterinary pharmacovigilance market is categorized into software and services. The software segment garnered the highest revenue share in the veterinary pharmacovigilance market in 2022. Over time, a lot more software applications have been embraced by diverse end consumers. Major drug makers are implementing these solutions in response to the growing demand for products and services in the pharmaceutical sector, which aims to reduce the likelihood of adverse drug reactions in animals. Particularly in affluent countries, these solutions are used more frequently than in developing nations.Product Outlook
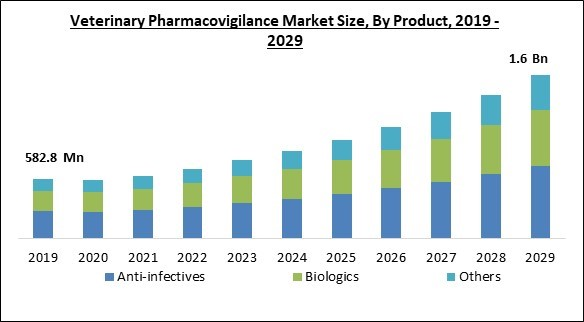
On the basis of product, the veterinary pharmacovigilance market is divided into biologics, anti-infectives, and others. The anti-infectives segment acquired the largest revenue share in the veterinary pharmacovigilance market in 2022. Anti-infectives were the products that received the greatest pharmacovigilance reports, which is why the segment is growing. External parasites, antimicrobials, internal parasites, and ectoparasiticides, for instance, were discovered to be among the most widely mentioned categories of products in pharmacovigilance reports, according to the 2021 Post-MA surveillance review by the French Agency for Food, Environmental, and Occupational Health and Safety (ANSES).Type Outlook
Based on type, the veterinary pharmacovigilance market is segmented into in-house and contract outsourcing. The contract outsourcing segment garnered a remarkable growth rate in the veterinary pharmacovigilance market in 2022. This results from the rise in animal health enterprises, the expansion of product approvals, and the outsourcing of pharmacovigilance functions by small and medium-sized animal health companies to save costs and time. Another major market force is participants' actions to expand their product offerings.Animal Type Outlook
By animal type, the veterinary pharmacovigilance market is classified into dogs, cats, and others. The cats segment acquired a substantial revenue share in the veterinary pharmacovigilance market in 2022. Cats lift their owners' moods and improve their emotional well-being. According to a study, cats typically improve their mood. Another benefit of these animals is that they promote older people's socializing. In addition, they aid in the socialization of those with mental or physical impairments. As a result many households have cats as pets, for example, cats are maintained as pets in about 40 million US homes.Regional Outlook
On the basis of region, the veterinary pharmacovigilance market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment recorded the highest revenue share in the veterinary pharmacovigilance market in 2022. The expanding regulatory framework that controls the administration of veterinary medications is one of the main motivators. For example, adverse event reports for veterinary medications can be sent electronically to the FDA's Center for Veterinary Medicine (CVM) using the Rational Questionnaire (RQ) in the Safety Reports Portal (SRP) as well as the Electronic Submissions System (ESS).The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Accenture PLC, ArisGlobal LLC, Ennov SAS, Sarjen Systems Pvt. Ltd., PharSafer Associates Ltd., Knoell Germany GmbH, biologit, Indivirtus Group (Indivirtus Healthcare Services), Oy Medfiles Ltd., and Azierta Life Sciences & Health Consulting Firm S.L.
Scope of the Study
By Product
- Anti-infectives
- Biologics
- Others
By Animal Type
- Dogs
- Cats
- Others
By Solution
- Software
- Services
By Type
- In-house
- Contract Outsourcing
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Accenture PLC
- ArisGlobal LLC
- Ennov SAS
- Sarjen Systems Pvt. Ltd.
- PharSafer Associates Ltd.
- Knoell Germany GmbH
- biologit
- Indivirtus Group (Indivirtus Healthcare Services)
- Oy Medfiles Ltd.
- Azierta Life Sciences & Health Consulting Firm S.L
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Accenture PLC
- ArisGlobal LLC
- Ennov SAS
- Sarjen Systems Pvt. Ltd.
- PharSafer Associates Ltd.
- Knoell Germany GmbH
- biologit
- Indivirtus Group (Indivirtus Healthcare Services)
- Oy Medfiles Ltd.
- Azierta Life Sciences & Health Consulting Firm S.L