Risk of Immunogenic Effects and Unsatisfactory ADME Properties
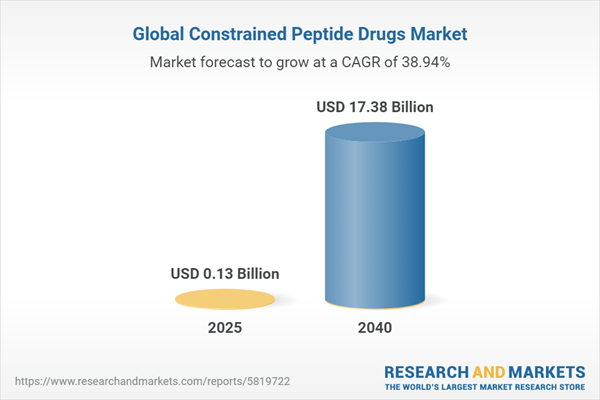
The global constrained peptide drugs market revenue has been forecasted from 2024 to 2040, following the earliest launch of the first constrained peptide drug in the market. The market size is anticipated to be $0.06 billion in 2024 and is expected to reach $17.38 billion in 2040, growing at a CAGR of 38.94% during the forecast period 2025-2040.
Market Lifecycle Stage
The global constrained peptide drugs market is anticipated to witness tremendous growth during the forecast period 2025-2040, largely fuelled by the promise of a novel breakthrough constrained peptide pipeline, which is no longer restricted to receptor targets. Advancements in chemical technologies, the therapeutics' success of commercialized synthetic peptides in recent years, and the affordable pricing being realized by these biomolecules in a wide range of diseases are some additional factors attributing to the projected growth in the forecast period.
Impact
The impact analysis for the factors that significantly affect the market, namely, drivers, restraints, and opportunities, has been provided on a short-term and long-term basis. The short-term assessment considers the period between 2020 and 2025, and the long-term assessment considers the period between 2026 and 2040. Key developments and strategies that have been undertaken by some of the key players in this market have been accounted for evaluation of the impact analysis. Further, these key developments have been assessed to understand the future scope of integrating advancing technologies to enable superior outcomes. Additionally, approvals and launches from companies and patent bodies have also been considered while evaluating the dynamics of the global constrained peptide drugs market.
Impact of COVID-19
In December 2019, Wuhan, a city in the Hubei region of China, was the site of the first detection of the COVID-19 outbreak. Following the classification of COVID-19 as novel pneumonia due to a cluster of unexplained pneumonia cases, efforts to pinpoint the culprit causing the outbreak and outline its genomic sequence got underway right once. The virus has already spread to every country on the globe, and researchers, governments, and business leaders are working to find answers to the crisis at a scale and speed that has never been seen. Testing for SARS-CoV-2 in the populace is one of the main steps that has been put into place globally, among many other measures used to stop the spread of the disease.
The global constrained peptide drugs market is a research-oriented market, having a majority of products in clinical trial stages. It primarily consists of clinical-stage biopharmaceutical companies and global biopharmaceutical companies such as Bicycle Therapeutics PLC, Protagonist Therapeutics, Inc., Aileron Therapeutics, Inc., Polyphor, and Santhera Pharmaceuticals Holding. Since most of the products are in the clinical phase of drug development, constrained peptide drug companies had a low impact on the COVID-19 pandemic.
Although clinical trials were brought to pause because of the lockdowns imposed by governments across the world, causing a delay in the clinical trial timeline, and volunteers and patients were also not able to participate in the clinical trials due to the lockdown; however, few companies took the opportunity of the pandemic and initiated the development of potential drugs against COVID-19. For instance, UCB Pharma participated in the COVID-19 Moonshot, an initiative to expedite the development of an anti-viral for COVID-19. The company’s Phase III investigational molecule Zilucoplan is being studied for acute respiratory distress syndrome associated with COVID-19. Another company, Polyphor Limited, is evaluating Balixafortide, a constrained peptide drug, against COVID-19 as it demonstrated strong efficacy in in-vitro models.
During the pre-COVID-19 period, the global constrained peptide drugs market observed 38 significant key developments. Out of the 38 key developments, the majority were funding activities, primarily focused on the development of novel antibiotics and support clinical trials of certain drugs in clinical phases. For example, in May 2019, Innosuisse awarded Polyphor Ltd. and the University of Zurich to escalate the development of novel antibiotics for treating infections caused by gram-negative bacteria. Furthermore, seven synergistic activities were undertaken in the global constrained peptide drugs market before COVID-19.
Market Segmentation
Segmentation 1: by Peptide Type
- Disulfide-Rich Peptides (DRPs)
- Cyclic Peptides
Based on peptide type, the disulfide-rich peptides (DRPs) segment is anticipated to dominate the global constrained peptide drugs market in 2040 as the segment includes the pipeline-constrained peptide with either limited existing treatment options or no approved drugs for the disease.
Segmentation 2: by Region
- North America
- Europe
- Asia-Pacific
The North America region is anticipated to dominate the global constrained peptide drugs market (by region) during the forecast period 2025-2040. The reasons contributing to the high demand for constrained peptide drugs in North America are the increasing prevalence of target indications and the early launch of pipeline products in the U.S. and Canada.
Segmentation 3: by Potential Product
- BT5528
- Rusfertide (PTG-300)
- PN-943
- PN-235
- Zilucoplan (RA101495)
Segmentation 4: by Company
- Aileron Therapeutics, Inc.
- Bicycle Therapeutics plc
- Spexis AG
- Protagonist Therapeutics Inc.
- Santhera Pharmaceuticals
- Union Chimique Belge S.A. (UCB)
- Creative Peptides
- Biosynth (Pepscan)
- Pepticom Ltd.
- PeptiDream, Inc.
- Bio-Synthesis Inc
- CPC Scientific Inc.
- Circle Pharma
- Zealand Pharma
- Chugai Pharmaceutical Co., Ltd.
Based on the company, the global constrained peptide drugs market is dominated by 15 major companies.
Recent Developments in the Global Constrained Peptide Drugs Market
- In August 2021, Protagonist Therapeutics Inc. declared the resolution of its collaboration agreement with Zealand Pharma through the reduction of future milestone payments, sales milestones, and royalties owed to Zealand Pharma regarding Protagonist Therapeutics Inc.’s product candidate rusfertide under the terms of the 2012 collaboration agreement between the companies.
- In April 2021, Union Chimique Belge S.A. (UCB) anticipated the release of phase 3 key results for generalized myasthenia gravis (gMG) in the fourth quarter of 2021 and discontinued further development of zilucoplan for immune-mediated necrotizing myopathy (IMNM).
- In September 2021, Polyphor Limited and EnBiotix announced the successful closing of the purchase agreement for EnBiotix to acquire the inhaled antibiotic murepavadin.
- In November 2020, Pepscan Therapeutics B.V. was granted a license for the use of the proprietary CLIPS technology offered by Bicycle Therapeutics plc. The peptide-constraining technology would further help in the development of the company’s products named BT1718 and THR-149.
- In June 2020, Santhera Pharmaceuticals secured a financing commitment of up to $22.1 million from a fund managed by Highbridge Capital Management.
Demand - Drivers and Limitations
The following are the demand drivers for the global constrained peptide drugs market:
- Enhanced Binding Affinity and Cellular Uptake
- Development of Synthetic Constraining Method
- Limitations with Conventional Peptides
- Increasing Government and Private Funding
The market is expected to face some limitations due to the following challenges:
- Increased Competition from Biologics
- Risk of Immunogenic Effects and Unsatisfactory ADME Properties
How can this report add value to an organization?
Workflow/Innovation Strategy: The global constrained peptide drugs market has been segmented (by product) into five candidates, i.e., BT5528, Rusfertide (PTG-300), Zilucoplan (RA101495), PN-235, and PN-943. Over the past decade, peptide drug discovery and development has witnessed a renaissance and scientific thrust as the industry has come to acknowledge the capability of peptide therapeutics in addressing unmet medical needs and the potential of this class of molecules to become a significant accompaniment or even favored alternative treatment to biologics and small molecules.
Peptide therapeutics have demonstrated a novel and selective yet safe mode of action for a wide range of indications. The existing and future development of constrained peptide drugs will continue to burgeon upon the strengths of constrained peptides and innovative technologies employed in the discovery and development, including peptide drug conjugates, multifunctional peptides, and cell-penetrating peptides. Furthermore, limitations associated with presently available peptides have resulted in an urgent need for new design, administration, and synthesis in peptide therapeutics, thereby leading to advancements in the development of constrained peptides.
Growth/Marketing Strategy: Constrained peptides provide noteworthy advantages over linear peptides. An increase in interest in the field of constrained peptides due to the properties they offer led to advancements in peptide synthesis technologies. Companies such as PeptiDream Inc., Pepticom Ltd., Bicycle Therapeutics plc, and Polyphor Limited offer proprietary drug development and constrained peptide synthesis technologies. PeptiDream Inc.’s proprietary Peptide Discovery Platform System (PDPS) technology is used to synthesize synthetic non-native peptide libraries expeditiously, which helps in identifying peptides that can be used as potential drugs for a disease.
Competitive Strategy: Key players in the global constrained peptide drugs market have been analyzed and profiled in the study, including manufacturers involved in new product development, acquisitions, expansions, and strategic collaborations. Moreover, a detailed competitive benchmarking of the players operating in the global constrained peptide drugs market has been done to help the reader understand how players stack against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Table of Contents
Companies Mentioned
- Aileron Therapeutics, Inc.
- Bicycle Therapeutics plc
- Spexis AG
- Protagonist Therapeutics Inc.
- Santhera Pharmaceuticals
- Union Chimique Belge S.A. (UCB)
- Creative Peptides
- Biosynth (Pepscan)
- Pepticom Ltd.
- PeptiDream, Inc.
- Bio-Synthesis Inc
- CPC Scientific Inc.
- Circle Pharma
- Zealand Pharma
- Chugai Pharmaceutical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 229 |
| Published | May 2023 |
| Forecast Period | 2025 - 2040 |
| Estimated Market Value ( USD | $ 0.13 Billion |
| Forecasted Market Value ( USD | $ 17.38 Billion |
| Compound Annual Growth Rate | 38.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |