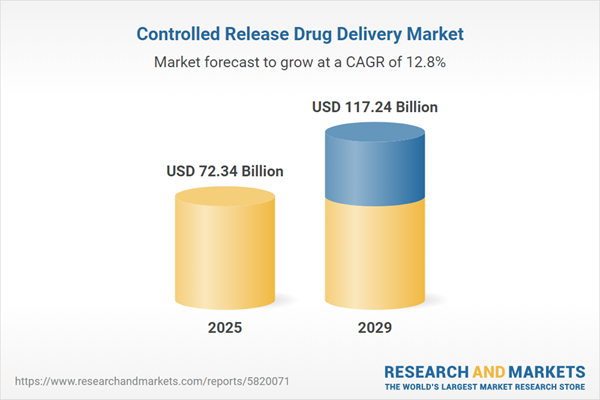
The controlled release drug delivery market size is expected to see rapid growth in the next few years. It will grow to $117.24 billion in 2029 at a compound annual growth rate (CAGR) of 12.8%. The growth in the forecast period can be attributed to shift towards personalized medicine, technological integration for patient convenience, expansion of biologics and biosimilars, market expansion in developing regions, advancements in polymer science. Major trends in the forecast period include biodegradable and biocompatible materials, nanotechnology advancements, personalized medicine and targeted therapies, advances in implantable devices, innovative encapsulation techniques.
The forecast of 12.8% growth over the next five years reflects a modest reduction of 0.3% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. The imposition of tariffs may pose a significant challenge for formulation scientists by increasing costs of specialized matrix polymers for controlled release systems sourced from Germany and Belgium, potentially delaying extended-release product launches and raising drug delivery technology expenditures. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The increasing prevalence of chronic diseases, also termed noncommunicable diseases (NCDs), is projected to act as a significant driver for the expansion of the controlled release drug delivery market. Chronic diseases, which encompass ailments such as heart disease, cancer, and diabetes, persist over extended periods, necessitating ongoing medical attention and often restricting daily activities. Controlled-release drug delivery plays a pivotal role in treating such chronic conditions. Particularly for patients requiring prolonged treatment or residing in areas with limited healthcare access, drug delivery formulations facilitating extended medication release are paramount. For instance, a September 2022 report shared by the World Health Organization (WHO) highlighted that annually, 41 million people succumb to chronic diseases, accounting for 74 percent of global fatalities. Approximately 17 million individuals lose their lives to chronic ailments before reaching the age of 70. Moreover, 77 percent of deaths attributed to chronic diseases occur in low- and middle-income countries, with anticipated future increases. Thus, the escalating prevalence of chronic diseases fuels the growth of the controlled release drug delivery market.
The rising healthcare expenditure is projected to drive the growth of the controlled release drug delivery market in the coming years. Healthcare spending encompasses all financial resources - both public and private - allocated to the healthcare sector within a specific geographic area, country, or economy during a designated time frame. Investment in controlled release drug delivery is a multifaceted endeavor that includes the entire lifecycle of a medicinal product, from initial research to post-market monitoring, aimed at enhancing treatment outcomes, patient adherence, and overall healthcare efficiency. For instance, in May 2024, the Office for National Statistics, a UK-based statistics authority, reported that healthcare expenditure in the UK was approximately £292 billion in 2023, marking a nominal increase of 5.6%. Furthermore, long-term health and social care spending experienced a real-term growth of 2.8% in 2022. Consequently, the increasing healthcare spending is driving the growth of the controlled release drug delivery market.
Technological progression stands out as a pivotal trend gaining traction within the controlled-release drug delivery market. Major enterprises operating in this sector are embracing novel technologies to fortify their market positions. For example, in March 2022, Evonik Industries AG, a Germany-based pharmaceutical company, introduced UDRATEC SoluFlow. This innovative microparticle technology is engineered to enhance the solubility of active medicinal constituents in oral medication formulations. Utilizing an emulsion-based processing technique, UDRATEC SoluFlow overcomes solubility barriers unattainable through traditional production methods. This addition to Evonik Health Care's suite of oral drug delivery system solutions complements existing offerings such as oral excipients such as EUDRAGIT functional polymers, ready-to-fill functional capsules such as EUDRACAPTM, and various technologies and services aimed at optimizing medication performance.
Leading entities within the chocolate syrup market are strategically engaging in partnership initiatives, with a focus on broadening applications in the ophthalmic field. Strategic partnerships denote a collaborative process wherein companies leverage each other's strengths and resources to achieve mutual benefits and foster success. For instance, in March 2023, Hovione, a Portugal-based contract drug manufacturing company, unveiled a collaboration with Ripple Therapeutics Inc. to expand the utilization of Ripple's Epidel technology beyond its current ophthalmic applications. This partnership marks a pivotal step in extending the Epidel platform's reach beyond ophthalmology, combining technical synergy, innovative vision, and a harmonious cultural alignment. Hovione's proficiency in regulated, sustained drug administration, coupled with their expertise in chemical synthesis and pharmaceutical manufacturing, forms a robust foundation for this collaboration. Ripple Therapeutics Inc. operates as a Canada-based pharmaceutical firm.
In November 2022, Alcon, a Switzerland-based pharmaceuticals and medical devices company, completed the acquisition of Aerie Pharmaceuticals, Inc. for an undisclosed sum. This strategic acquisition signifies an expansion of Alcon's diverse range of commercial products and development pipeline within the realm of ophthalmic pharmaceuticals. Aerie Pharmaceuticals, Inc., an esteemed US-based company specializing in the manufacture of ophthalmic solutions, further bolsters Alcon's portfolio and advancements in the ophthalmic pharmaceutical sector through this acquisition.
Major companies operating in the controlled release drug delivery market include Johnson & Johnson Services Inc., Merck & Co. Inc., Alkermes plc, Coating Place Inc., Corium International Inc., Depomed Inc., Pfizer Inc., Aradigm Corporation, Capsugel LLC, GlaxoSmithKline plc, Bayer Healthcare LLC, Collegium Pharmaceuticals Inc., GE HealthCare Technologies Inc., Adare Pharmaceuticals Inc., Allergan plc, Novartis AG, Lonza Group AG, Colorcon Inc., SKY Pharmaceuticals Pvt. Ltd., AstraZeneca plc, Biogen Inc., Orbis Biosciences Inc., Alza Corporation, MicroCHIPS Inc., Endo International plc, Heron Therapeutics Inc., Intellipharmaceutics International Inc., Ipsen Biopharmaceuticals Inc., Luye Pharma Group Ltd., Medtronic plc, Nektar Therapeutics, Novo Nordisk A/S, Ocular Therapeutix Inc., Pacira BioSciences Inc., Progenity Inc., Sanofi S.A., Teva Pharmaceutical Industries Ltd., Titan Pharmaceuticals Inc., Vectura Group plc, West Pharmaceutical Services Inc., Zosano Pharma Corporation, Zynerba Pharmaceuticals Inc.
North America was the largest region in the controlled release drug delivery market in 2024. The regions covered in the controlled release drug delivery market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the controlled release drug delivery market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The controlled release drug delivery market research report is one of a series of new reports that provides controlled release drug delivery market statistics, including controlled release drug delivery industry global market size, regional shares, competitors with a controlled release drug delivery market share, detailed controlled release drug delivery market segments, market trends and opportunities, and any further data you may need to thrive in the controlled release drug delivery industry. This controlled release drug delivery market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Controlled release drug delivery involves a dosage form designed to administer a medicine or medication in a specific manner, providing continuous and predictable kinetics for a predetermined duration.
The primary types of technology employed in controlled release drug delivery include the wurster technique, coacervation, microencapsulation, implants, transdermal delivery, targeted delivery, and others. The wurster technique, also known as fluid bed microencapsulation, encapsulates discrete particles in a fluidized bed using differential air flow to create cyclic movement of material. It encompasses various release mechanisms such as polymer-based systems, micro-reservoir partition-controlled drug delivery systems, feedback-regulated drug delivery systems, activation-modulated drug delivery systems, and chemically activated methods. These technologies find applications in metered dose inhalers, injectables, transdermal and ocular patches, among others. Major end-users include hospitals, clinics, individuals, research institutions, and others.
The controlled release drug delivery market consists of revenues earned by entities by providing services such as mucoadhesive drug delivery, implantable drug delivery, and gastro-retentive drug delivery. The market value includes the value of related goods sold by the service provider or included within the service offering. The controlled release drug delivery market also includes sales of nebulizers, infusion pumps, and other systems used to provide controlled release drug delivery. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Controlled Release Drug Delivery Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on controlled release drug delivery market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for controlled release drug delivery? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The controlled release drug delivery market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Technology: Wurster Technique; Coacervation; Micro Encapsulation; Implants; Transdermal; Targeted Delivery; Other Technologies2) By Release Mechanism: Polymer Based Systems; Micro Reservoir Partition Controlled Drug Delivery Systems; Feedback Regulated Drug Delivery Systems; Activation-Modulated Drug Delivery Systems; Chemically Activated;
3) By Application: Metered Dose Inhalers; Injectable; Transdermal and Ocular Patches; Other Applications
4) By End User: Hospitals; Clinics; Personal; Research; Other End Users
Subsegments:
1) By Wurster Technique: Spray Coating; Fluid Bed Coating2) By Coacervation: Simple Coacervation; Complex Coacervation
3) By Micro Encapsulation: Polymer-Based Micro Encapsulation; Lipid-Based Micro Encapsulation
4) By Implants: Biodegradable Implants; Non-Biodegradable Implants
5) By Transdermal: Microneedle Patches; Reservoir Systems; Matrix Systems
6) By Targeted Delivery: Nanoparticles; Antibody-Drug Conjugates; Liposomes
7) By Other Technologies: Osmotic Pumps; in Situ Gelling Systems
Companies Mentioned: Johnson & Johnson Services Inc.; Merck & Co. Inc.; Alkermes plc; Coating Place Inc.; Corium International Inc.; Depomed Inc.; Pfizer Inc.; Aradigm Corporation; Capsugel LLC; GlaxoSmithKline plc; Bayer Healthcare LLC; Collegium Pharmaceuticals Inc.; GE HealthCare Technologies Inc.; Adare Pharmaceuticals Inc.; Allergan plc; Novartis AG; Lonza Group AG; Colorcon Inc.; SKY Pharmaceuticals Pvt. Ltd.; AstraZeneca plc; Biogen Inc.; Orbis Biosciences Inc.; Alza Corporation; MicroCHIPS Inc.; Endo International plc; Heron Therapeutics Inc.; Intellipharmaceutics International Inc.; Ipsen Biopharmaceuticals Inc.; Luye Pharma Group Ltd.; Medtronic plc; Nektar Therapeutics; Novo Nordisk a/S; Ocular Therapeutix Inc.; Pacira BioSciences Inc.; Progenity Inc.; Sanofi S.A.; Teva Pharmaceutical Industries Ltd.; Titan Pharmaceuticals Inc.; Vectura Group plc; West Pharmaceutical Services Inc.; Zosano Pharma Corporation; Zynerba Pharmaceuticals Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Controlled Release Drug Delivery market report include:- Johnson & Johnson Services Inc.
- Merck & Co. Inc.
- Alkermes plc
- Coating Place Inc.
- Corium International Inc.
- Depomed Inc.
- Pfizer Inc.
- Aradigm Corporation
- Capsugel LLC
- GlaxoSmithKline plc
- Bayer Healthcare LLC
- Collegium Pharmaceuticals Inc.
- GE HealthCare Technologies Inc.
- Adare Pharmaceuticals Inc.
- Allergan plc
- Novartis AG
- Lonza Group AG
- Colorcon Inc.
- SKY Pharmaceuticals Pvt. Ltd.
- AstraZeneca plc
- Biogen Inc.
- Orbis Biosciences Inc.
- Alza Corporation
- MicroCHIPS Inc.
- Endo International plc
- Heron Therapeutics Inc.
- Intellipharmaceutics International Inc.
- Ipsen Biopharmaceuticals Inc.
- Luye Pharma Group Ltd.
- Medtronic plc
- Nektar Therapeutics
- Novo Nordisk A/S
- Ocular Therapeutix Inc.
- Pacira BioSciences Inc.
- Progenity Inc.
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
- Titan Pharmaceuticals Inc.
- Vectura Group plc
- West Pharmaceutical Services Inc.
- Zosano Pharma Corporation
- Zynerba Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 72.34 Billion |
| Forecasted Market Value ( USD | $ 117.24 Billion |
| Compound Annual Growth Rate | 12.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 43 |