Cardiac point-of-care (PoC) testing devices are innovative and portable medical instruments designed for the rapid and accurate diagnosis of cardiac conditions. They are compact, user-friendly, and offer real-time results, enabling physicians to make prompt and informed decisions about patient care. They provide healthcare professionals with the ability to conduct diagnostic tests at the bedside of patients, eliminate the need for laboratory-based testing, and reduce turnaround times for critical cardiac assessments. They enhance the efficiency of clinical workflows by delivering accurate and reliable results within minutes. They assist in performing a variety of diagnostic tests, including cardiac biomarker analysis, coagulation monitoring, cholesterol profiling, and electrolyte measurement. They aid in obtaining immediate test results that facilitate more efficient patient triage, reduce overcrowding in emergency departments, and optimize resource allocation. As they minimize costs associated with sample transportation, lab personnel, and infrastructure by reducing the reliance on centralized laboratories, the demand for cardiac PoC testing devices is rising worldwide.
Cardiac PoC Testing Devices Market Trends:
At present, the rising prevalence of cardiovascular diseases due to the excessive consumption of tobacco and alcohol represents one of the crucial factors bolstering the growth of the market. Moreover, there is an increase in the utilization of cardiac PoC testing devices to detect myocardial infarction and coronary syndromes around the world. This, along with the thriving medical industry, is supporting the growth of the market. In addition, the growing employment of these devices to identify brain natriuretic peptides, kinase, synthesizing troponin, and lactate dehydrogenase isoenzymes that are generated during heart failure is positively influencing the market. Besides this, key players are focusing on launching smartphone-based tests for cardiovascular diseases. They are also developing technologically advanced cardiac biomarker analyzers, which is bolstering the growth of the market. Additionally, there is a rise in the demand for cardiac analyzers, as they have software to provide an internal standard to account for membrane variability that is intrinsic to tests and integrated LCD displays for user convenience and visual clarity. This, coupled with the ability of analyzers to quantify the concentration of biomarkers in whole blood, serum, and plasma, which helps in clinical diagnosis, is impelling the growth of the market. Furthermore, the growing technological advancements in PoC testing devices are offering a favorable market outlook.Key Market Segmentation:
The publisher provides an analysis of the key trends in each segment of the global cardiac PoC testing devices market, along with forecasts at the global, regional, and country levels from 2025-2033. Our report has categorized the market based on product type and end user.Product Type Insights:
- Cardiac Markers Test
- Cardiac Troponin (cTn) Test
- Myoglobin Test
- Creatine Kinase MB Isoenzyme (CK-MB) Test
- Combinational Test Kits
- Brain Natriuretic Peptide (BNP) Test
- Analyzers
End User Insights:
- Hospitals
- Diagnostic Laboratories
- Others
Regional Insights:
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
Competitive Landscape:
The report has also provided a comprehensive analysis of the competitive landscape in the global cardiac PoC testing devices market. Detailed profiles of all major companies have been provided. Some of the companies covered include Abbott Laboratories, ACON Laboratories Inc., Alfa Scientific Designs Inc., Bio-Rad Laboratories Inc., Danaher Corporation, F. Hoffmann-La Roche AG, LifeSign LLC, Nano-Ditech Corporation, Nexus-Dx Inc., etc. Kindly note that this only represents a partial list of companies, and the complete list has been provided in the report.Key Questions Answered in This Report:
- How has the global cardiac PoC testing devices market performed so far, and how will it perform in the coming years?
- What are the drivers, restraints, and opportunities in the global cardiac PoC testing devices market?
- What is the impact of each driver, restraint, and opportunity on the global cardiac PoC testing devices market?
- What are the key regional markets?
- Which countries represent the most attractive cardiac PoC testing devices market?
- What is the breakup of the market based on the product type?
- Which is the most attractive product type in the cardiac PoC testing devices market?
- What is the breakup of the market based on the end user?
- Which is the most attractive end user in the cardiac PoC testing devices market?
- What is the competitive structure of the global cardiac PoC testing devices market?
- Who are the key players/companies in the global cardiac PoC testing devices market?
Table of Contents
Companies Mentioned
- Abbott Laboratories
- ACON Laboratories Inc.
- Alfa Scientific Designs Inc.
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- F. Hoffmann-La Roche AG
- LifeSign LLC
- Nano-Ditech Corporation
- Nexus-Dx Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 136 |
| Published | March 2025 |
| Forecast Period | 2024 - 2033 |
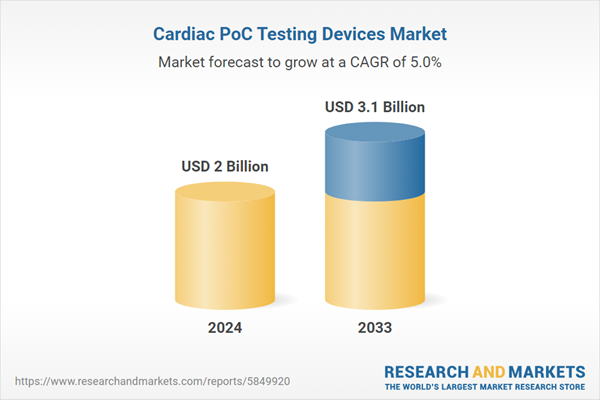
| Estimated Market Value ( USD | $ 2 Billion |
| Forecasted Market Value ( USD | $ 3.1 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |