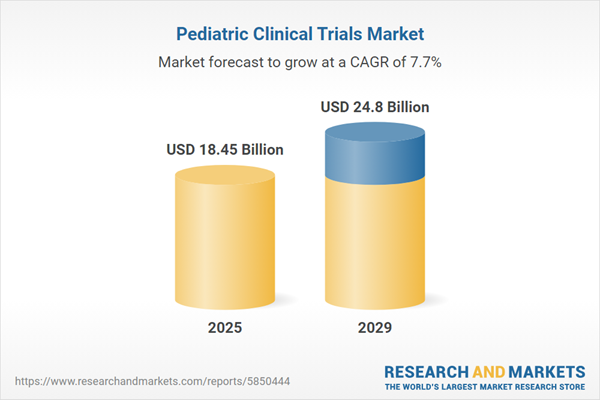
The pediatric clinical trials market size is expected to see strong growth in the next few years. It will grow to $24.8 billion in 2029 at a compound annual growth rate (CAGR) of 7.7%. The growth in the forecast period can be attributed to integration of real-world evidence, pediatric biomarker discovery, expanded access programs, pediatric infectious disease research, pediatric cardiology advancements. Major trends in the forecast period include patient-centric trial design, adoption of adaptive trial designs, digital health technologies, global collaboration and consortia, pediatric oncology advancements.
The rise in pediatric cancer cases is expected to drive the pediatric clinical trials market forward. Pediatric cancers, though rare, exhibit unique growth patterns distinct from adult tumors, necessitating specialized research and treatments. Pediatric clinical trials play a pivotal role in advancing prognosis and treatment methods. According to the American Cancer Society's estimates in January 2023, approximately 9,910 children in the US under 15 will be diagnosed with cancer, with about 1,040 expected deaths in 2023. Consequently, increased healthcare spending fuels the growth of the pediatric clinical trials market.
The burgeoning demand for outsourcing services is set to propel the pediatric clinical trials market's expansion. As preclinical research grows in complexity, pharmaceutical and biotech companies increasingly seek specialized skills beyond their in-house capabilities. Outsourcing services aid in crafting safe, ethical, and scientifically robust pediatric clinical trial protocols. A survey by BioPlan Associates Inc. in April 2023 revealed that around 84.6% of biomanufacturers outsource analytical testing, while 74.5% seek toxicity testing outsourcing, highlighting the surging reliance on outsourcing in pediatric clinical trials, driving market growth.
Product innovation stands out as a pivotal trend reshaping the pediatric clinical trial market. Companies within this sector are investing in innovative products to maintain their market standing. An instance is The Leukemia & Lymphoma Society's launch of the Pediatric Acute Leukemia (PedAL) Master Clinical Trial in June 2022. This groundbreaking trial is tailored to pediatric acute leukemia, leveraging individual tumor biology to match children with the most promising treatments, emphasizing innovation in pediatric cancer research.
Major players in pediatric clinical trials prioritize collaborations and partnerships to deliver dependable services. Strategic partnerships, characterized by structured affiliations between commercial entities, are pivotal in fostering reliability in service provision. Strados Labs' collaboration with Ann & Robert H. Lurie Children's Hospital in September 2023 exemplifies this approach. Their clinical trial involves the RESP Biosensor, a wearable device employing acoustic sensors to monitor lung sounds for asthma symptoms. The trial aims to assess the RESP Biosensor's effectiveness in accurately detecting and monitoring asthma exacerbations in children, showcasing partnerships' role in advancing pediatric healthcare technology.
In March 2024, PCM Trials, a U.S.-based provider of mobile research nurse visits for decentralized clinical trials, acquired EmVenio Research for an undisclosed amount. This acquisition enables PCM Trials to adopt a more patient-centric and decentralized approach to clinical trials, enhancing participant recruitment and retention, especially among underrepresented minorities. EmVenio is a U.S.-based company focused on improving access to pediatric clinical trials, particularly for diverse and underserved populations.
Major companies operating in the pediatric clinical trials market include Medpac Inc., Pharmaceutical Product Development Inc., ICON plc, Syneos Health Inc., QPS Holdings LLC, Pfizer Inc., IQVIA Inc., Premier Research, Labcorp - Laboratory Corporation of America Holdings, The Emmes Company LLC, Synteract Inc., Charles River Laboratories International Inc., Covance Inc., Bristol-Myers Squibb Company, GlaxoSmithKline plc, BioNTech SE, Moderna Inc., Parexel International Corporation, PRA Health Sciences, Wuxi AppTec, Merck & Co. Inc., AstraZeneca plc, Sanofi S.A., Johnson & Johnson, Eli Lilly and Company, Takeda Pharmaceutical Company Limited, C.H. Boehringer Sohn AG & Co. KG, AbbVie Inc., Teva Pharmaceutical Industries Ltd., Oracle Health sciences.
North America was the largest region in the pediatric clinical trials market in 2024. The regions covered in the pediatric clinical trials market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the pediatric clinical trials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Pediatric clinical trials serve as critical investigations into the efficacy, safety, and appropriate dosage of pharmaceuticals, medical devices, and therapies specifically tailored for children. Developing age-appropriate treatments and procedures is paramount in refining and identifying the most effective medical interventions available for pediatric patients.
These trials typically encompass several distinct phases Phase I, Phase II, Phase III, and Phase IV. Phase I trials primarily concentrate on establishing the safety profile and determining the appropriate dosage range of new medications, typically involving 20-100 healthy volunteers. Diverse study designs, such as treatment and observational studies, are employed across various therapeutic areas. These encompass infectious diseases, oncology, autoimmune or inflammatory diseases, respiratory disorders, mental health disorders, among other medical domains, enabling comprehensive exploration and assessment of treatments for pediatric patients.
The pediatric clinical trials market research report is one of a series of new reports that provides pediatric clinical trials market statistics, including pediatric clinical trials industry global market size, regional shares, competitors with a pediatric clinical trials market share, detailed pediatric clinical trials market segments, market trends and opportunities, and any further data you may need to thrive in the pediatric clinical trials industry. This pediatric clinical trials market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The pediatric clinical trials market includes revenues earned by entities through clinical research services, consulting, outsourcing services, and medical communications. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Pediatric Clinical Trials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pediatric clinical trials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pediatric clinical trials? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The pediatric clinical trials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Phase: Phase I; Phase II; Phase III; Phase IV2) By Study Design: Treatment Studies; Observational Studies
3) By Therapeutic Areas: Infectious Diseases; Oncology; Autoimmune or Inflammatory Diseases; Respiratory Disorders; Mental Health Disorders; Other Therapeutic Areas
Subsegments:
1) By Phase I: First-in-Pediatrics Trials; Dose Escalation Studies; Safety and Tolerability Assessments2) By Phase II: Efficacy Studies; Dose Optimization Trials; Pharmacokinetic Studies
3) By Phase III: Large-Scale Efficacy Trials; Comparative Studies; Long-Term Safety Studies
4) By Phase IV: Post-Marketing Surveillance; Long-Term Safety Monitoring; Effectiveness Studies in Real-World Settings
Key Companies Mentioned: Medpac Inc.; Pharmaceutical Product Development Inc.; ICON plc; Syneos Health Inc.; QPS Holdings LLC
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Medpac Inc.
- Pharmaceutical Product Development Inc.
- ICON plc
- Syneos Health Inc.
- QPS Holdings LLC
- Pfizer Inc.
- IQVIA Inc.
- Premier Research
- Labcorp - Laboratory Corporation of America Holdings
- The Emmes Company LLC
- Synteract Inc.
- Charles River Laboratories International Inc.
- Covance Inc.
- Bristol-Myers Squibb Company
- GlaxoSmithKline plc
- BioNTech SE
- Moderna Inc.
- Parexel International Corporation
- PRA Health Sciences
- Wuxi AppTec
- Merck & Co. Inc.
- AstraZeneca plc
- Sanofi S.A.
- Johnson & Johnson
- Eli Lilly and Company
- Takeda Pharmaceutical Company Limited
- C.H. Boehringer Sohn AG & Co. KG
- AbbVie Inc.
- Teva Pharmaceutical Industries Ltd.
- Oracle Health sciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 18.45 Billion |
| Forecasted Market Value ( USD | $ 24.8 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |