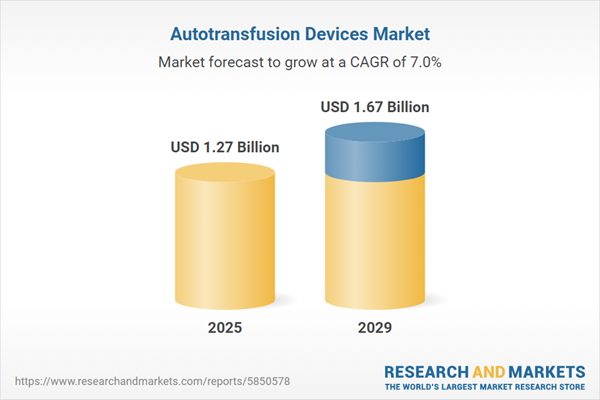
The autotransfusion devices market size is expected to see strong growth in the next few years. It will grow to $1.67 billion in 2029 at a compound annual growth rate (CAGR) of 7%. The growth in the forecast period can be attributed to the growing geriatric population, expansion of ambulatory surgical centers, focus on blood conservation in obstetrics, and adoption in developing healthcare markets. Major trends in the forecast period include technological advancements in blood processing, integration of advanced sensors, customization for different procedures, single-donor closed-loop systems, and miniaturization of devices.
The anticipated rise in cardiovascular diseases (CVDs) is set to propel growth within the autotransfusion device market. CVDs encompass a spectrum of heart and blood vessel disorders, and autotransfusion devices play a crucial role in reducing reliance on allogeneic blood transfusions. These devices facilitate reinfusion of a patient's own blood, mitigating the risks associated with donor blood transfusions. Notably, the American Heart Association's Heart Disease and Stroke Statistics-2022 Update report highlighted CVDs as the foremost global cause of mortality, claiming approximately 17.9 million lives annually, underscoring the market's trajectory.
The escalating demand for blood conservation is expected to be a significant driver for the autotransfusion devices market. Within surgical contexts, blood conservation aims to avoid allogeneic blood transfusions during and post-surgery to enhance patient outcomes and safeguard their well-being. Autotransfusion devices effectively minimize blood loss during surgical procedures, contributing to improved patient recovery. As per the June 2023 report by the World Health Organization, a substantial percentage of reporting countries, accounting for 73% or 125 out of 171, have implemented national blood policies. This indicates a widespread initiative toward blood conservation, affirming the market's growth.
The rising number of orthopedic surgeries is anticipated to fuel the growth of the autotransfusion devices market. Orthopedic surgeries involve medical procedures that address issues related to the musculoskeletal system, which includes bones, joints, ligaments, tendons, and muscles. The increase in these surgeries can be linked to a growing demand for treatments for injuries and degenerative conditions, as well as an aging population that requires joint replacements and repairs. Autotransfusion devices play a crucial role in orthopedic surgeries by collecting, processing, and reinfusing a patient’s blood lost during the operation, thereby reducing the need for donor blood and minimizing the risk of transfusion-related complications. For example, the British Orthopaedic Association reported that in May 2022, England experienced over 42,000 orthopedic surgeries in March 2022, marking the highest number since June 2021. Consequently, the rising frequency of orthopedic surgeries is driving demand for autotransfusion devices.
Leading companies in the autotransfusion devices market are concentrating on creating technologically advanced solutions, including next-generation intelligent control software designed to improve precision, efficiency, and patient outcomes during blood transfusions. This next-generation software utilizes sophisticated algorithms to optimize the recovery, processing, and reinfusion of blood in real time, thereby enhancing both efficiency and patient safety. For example, in March 2023, Haemonetics Corporation, a U.S.-based provider of blood and plasma supplies and services, announced that the Cell Saver Elite+ Autotransfusion System received clearance from the U.S. Food and Drug Administration (FDA). This next-generation software maximizes the recovery of red blood cells during surgical procedures where medium to high blood loss is anticipated. This innovative upgrade streamlines autotransfusion processes, minimizes unnecessary allogeneic transfusions, and ultimately leads to improved patient outcomes.
Innovations in product development, particularly the integration of next-generation intelligent control software, signify a pivotal focus among major autotransfusion device companies. This software upgrade, known as Intelligent Control, introduces significant enhancements aimed at simplifying operations, thereby enhancing efficiency and user experience. For instance, Haemonetics, a US-based healthcare company, received FDA 510(k) clearance for its next-generation Intelligent Control software in March 2023, specifically designed for the Cell Saver Elite+ autotransfusion system. This advancement in software technology reaffirms Haemonetics' commitment to pioneering solutions for improved patient outcomes in surgeries involving medium to high blood loss scenarios, spanning cardiac, orthopedic, trauma, transplant, vascular, and OBGYN surgeries.
Major companies operating in the autotransfusion devices market include Becton Dickinson And Company, Medtronic plc, LivaNova plc, Haemonetics Corporation, Fresenius SE & Co KGaA, Terumo Corporation, Asahi Kasei Medical Co. Ltd., B. Braun Melsungen AG, GE HealthCare Technologies Inc., Cerus Corporation, Stryker Corporation, Atrium Medical Technologies, Brightwake Ltd., Getinge AB, Beijing ZKSK Technology Co. Ltd., Braile Biomédica, Redax S.p.A., Teleflex Incorporated, Zimmer Biomet Holdings Inc., Gen World Medical Devices Corporation, Soma Technology International LLC, Beijing Jingjing Medical Equipment Co. Ltd., Johnson & Johnson Inc., Sorin Group, Baxter International Inc., Dideco, Nipro Corporation.
North America was the largest region in the autotransfusion devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the global autotransfusion devices market during the forecast period. The regions covered in the autotransfusion devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the autotransfusion devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Autotransfusion devices are tools utilized to collect and reintroduce a patient's own blood during medical procedures, reducing reliance on donated blood - a method known as autologous blood transfusion. This approach notably diminishes the necessity for allogeneic (from a separate donor) blood transfusions. These devices find widespread application in trauma care within emergency departments and surgical settings.
The primary components of autotransfusion devices encompass autotransfusion systems, consumables, and accompanying accessories. Autotransfusion systems encompass both medical devices and methodologies enabling patients to receive their own blood for transfusions, negating the need for external donor blood. They serve diverse medical applications such as cardiovascular, orthopedic, neurological, obstetrics, gynecology surgeries, among others, and cater to various end-users including hospitals, specialized clinics, and other medical facilities.
The autotransfusion devices market research report is one of a series of new reports that provides autotransfusion devices optical components market statistics, including autotransfusion devices optical components industry global market size, regional shares, competitors with a autotransfusion devices optical components market share, detailed autotransfusion devices optical components market segments, market trends and opportunities, and any further data you may need to thrive in the autotransfusion devices optical components industry. This autotransfusion devices optical components market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The autotransfusion devices market consists of sales of on-pump transfusion devices and off-pump transfusion devices. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Autotransfusion Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on autotransfusion devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for autotransfusion devices? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The autotransfusion devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product Type: Autotransfusion Systems; Consumables and Accessories2) By Application: Cardiovascular Surgeries; Orthopedic Surgeries; Neurological Surgeries; Obstetrics and Gynecology Surgeries; Other Applications
3) By End-Use: Hospitals; Specialty Clinics; Other End Users
Subsegments:
1) By Autotransfusion Systems: Surgical Autotransfusion Systems; Emergency Autotransfusion Systems; Continuous Flow Autotransfusion Systems2) By Consumables and Accessories: Collection Reservoirs; Blood Filters; Tubing Sets; Pumps; Anticoagulants
Key Companies Mentioned: Becton Dickinson and Company; Medtronic plc; LivaNova plc; Haemonetics Corporation; Fresenius SE & Co KGaA
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Becton Dickinson And Company
- Medtronic plc
- LivaNova plc
- Haemonetics Corporation
- Fresenius SE & Co KGaA
- Terumo Corporation
- Asahi Kasei Medical Co. Ltd.
- B. Braun Melsungen AG
- GE HealthCare Technologies Inc.
- Cerus Corporation
- Stryker Corporation
- Atrium Medical Technologies
- Brightwake Ltd.
- Getinge AB
- Beijing ZKSK Technology Co. Ltd.
- Braile Biomédica
- Redax S.p.A.
- Teleflex Incorporated
- Zimmer Biomet Holdings Inc.
- Gen World Medical Devices Corporation
- Soma Technology International LLC
- Beijing Jingjing Medical Equipment Co. Ltd.
- Johnson & Johnson Inc.
- Sorin Group
- Baxter International Inc.
- Dideco
- Nipro Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.27 Billion |
| Forecasted Market Value ( USD | $ 1.67 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |