A sophisticated technique for identifying chromosomal abnormalities developing in the baby is non-invasive prenatal testing (NIPT). Currently, NIPT is regarded as an important test for expecting mothers to provide highly effective and affordable early identification of genetic diseases. The expansion of insurance coverage and reimbursement rules around the world is blamed for the market's expansion. The rising prevalence of genetic abnormalities and the healthcare industry's ongoing large-scale investments in genome sequencing are the key drivers of the growth of the worldwide NIPT market.
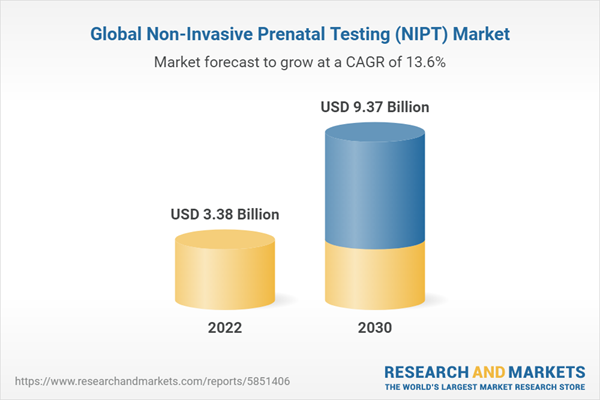
The non-invasive Prenatal Testing (NIPT) Market is predicted to expand at a CAGR of 13.60% during 2022-2030.
Prenatal screening has shifted to non-invasive methods in recent years owing to therapeutic and economic advantages. This trend is being pushed by sophisticated non-invasive diagnostics that have proven clinically useful. Non-invasive prenatal testing is becoming the norm for pregnant women in nations such as the Netherlands. Though intrusive diagnostics are still important for certain conditions, worldwide acceptance of non-invasive technologies has grown substantially. The rising frequency of chromosomal abnormalities in late pregnancies has raised the need for non-invasive fetal diagnostics. This demand is fueled even further by appealing reimbursement alternatives. Due to an increase in stillbirths, the market for these diagnostics is rapidly expanding, giving an opportunity for early illness detection.Advances in genomic sequencing technology, notably Next-generation sequencing (NGS), have reduced diagnostic time and complexity, leading in a rise in non-invasive prenatal testing (NIPT) using NGS technology. Life science companies are working hard to create NGS-based solutions for the early and accurate diagnosis of genetic disorders, which will help them compete in the market. Growing need for early and non-invasive fetal diagnostics, as well as favorable reimbursement regulations, are driving market expansion. Stillbirths also allow for early illness detection. According to study from April 2022, 0.4% to 0.9% of babies have chromosomal abnormalities, with the third most common autosomal trisomy affecting 1 in 5000 to 1 in 16000 live births. These situations raise testing demand, which supports total market development. In 2022, the market for non-invasive prenatal testing (NIPT) was worth US$3.38 Billion.
Cell-free DNA tests are growing in the worldwide NIPT market due to its non-invasive nature, which decreases risks and inconvenience for pregnant women.
Ultrasound detection, biochemical screening tests, cell-free DNA in maternal plasma tests, and others are among the procedures used in the worldwide non-invasive prenatal testing (NIPT) market. Because of advancements in sequencing technology and analytics, very accurate cell-free DNA tests for diagnosing chromosomal abnormalities are becoming more commonly accessible. Furthermore, rising mother age and the tendency toward later pregnancies have raised need for prenatal screening. Furthermore, the support and recognition of these tests by healthcare authorities and professional societies have further boosted their adoption, contributing to market growth.Next-generation sequencing (NGS) systems lead the global Non-invasive Prenatal Testing (NIPT) market.
ELISA Kits (Enzyme-Linked Immunosorbent Assay Kits), next-generation sequencing systems, and Microarray Analysis comprise the global Non-invasive Prenatal Testing (NIPT) market. NGS systems provide precise and accurate detection of fetal chromosomal abnormalities by sequencing large amounts of DNA. They are scalable to meet the increasing demand for NIPT, and their cost-effectiveness makes them preferable to other methods. NGS systems are widely available, improving access for pregnant women. Furthermore, NGS technology enables the development of new NIPT tests that detect a broader range of abnormalities, driving market growth.In comparison to other techniques like NGS systems, ELISA kits are a generally accessible and affordable choice for NIPT. They are useful for point-of-care testing and smaller laboratories since they are comparatively simple. Trisomy 21 and trisomy 18 are two frequent fetal chromosomal disorders that ELISA tests are highly accurate at identifying. A greater spectrum of abnormalities is detected by ELISA-based NIPT tests, fostering continued market expansion.
The dominance of the high- and average-risk segments in the global Non-invasive Prenatal Testing (NIPT) market is due to their specific needs.
Based on Risk Types, the global Non-invasive Prenatal Testing (NIPT) market is fragmented into high, average, and Low risks. High-risk pregnancies require accurate and comprehensive screening, while average-risk pregnancies benefit from non-invasive options. Advancements in technology, increased awareness, and supportive policies have driven the popularity of NIPT. Next-generation sequencing and other advanced techniques have improved accuracy and accessibility. The growing trend of personalized medicine and the desire for informed decision-making during pregnancy have also contributed to the demand for NIPT in both segments.The demand for NIPT is fueled by the assurance and early knowledge that expectant parents in low-risk pregnancies seek. NIPT has gained popularity due to technological development and public awareness, with the benefits of non-invasiveness, ease, and safety. Adoption of NIPT is further accelerated in the low-risk group by the move toward customized therapy and helpful reimbursement regulations.
Diagnostic centers experience significant growth in the global Non-invasive Prenatal Testing (NIPT) market due to their specialized expertise and equipment.
By End-User, the global Non-invasive Prenatal Testing (NIPT) market is divided into Specialty Centers, Hospitals, and Diagnostic Centers. Diagnostic centers offer comprehensive diagnostic services, including NIPT, as a convenient one-stop solution for prenatal care. Collaborations with healthcare providers and hospitals attract more patients. Personalized counseling and support enhance the patient experience. Increasing NIPT demand, awareness, investments, and supportive reimbursement policies drive diagnostic centers' expansion.The United States has a well-established market for Non-invasive Prenatal Testing (NIPT) tests.
United States, Canada, France, Germany, Italy, Spain, United Kingdom, Belgium, Netherlands, Switzerland, China, Japan, India, Australia, South Korea, Thailand, Malaysia, Indonesia, Philippines, Brazil, Mexico, Argentina, South Africa, Saudi Arabia, Turkey, U.A.E., and ROW are some of the top countries in the global Non-invasive Prenatal Testing (NIPT) market. The vast and varied patient population of the nation offers a large consumer base for NIPT testing. The United States' large healthcare expenditures make NIPT testing widely accessible. The adoption of NIPT testing is made easier by the favorable regulatory environment, which is supported by clear norms and regulations. Leading businesses and academic institutions are pushing innovation in the NIPT sector in the United States, which also has a robust ecosystem for research and development in the sector.The NIPT market has expanded as a result of the United States' culture of innovation and entrepreneurship, which has also made it more accessible and inexpensive for expectant mothers. Notable businesses involved in the growth and development of the United States NIPT market include Natera, Sequenom, and Ariosa Diagnostics.
Key Players
The leading rivals in the global Non-invasive Prenatal Testing (NIPT) market include F. Hoffman-La Roche, Eurofins Scientific, Illumina, Natera Inc., Revvity Inc., Thermo Fisher Scientific, Quest Diagnostics, and Agilent Technologies.In August 2022- As part of the Q-Sub procedure, Natera Inc. submitted a pre-submission to the Food and Drug Administration (FDA) for its panoramic non-invasive prenatal test (NIPT). For 22q11.2 deletion syndrome and fetal chromosomal aneuploidies, the business submitted its pre-submission in June 2022.
This latest report “Global Non-invasive Prenatal Testing (NIPT) Market, Method (ultrasound detection, biochemical screening tests, cell-free DNA in maternal plasma tests, and others), Technology (ELISA Kits (Enzyme-Linked Immunosorbent Assay Kits), Next Generation Sequencing Systems, and Microarray Analysis), Risk Type (High & Average risk, and Low risk), End-User (Specialty Centers, Hospitals, and Diagnostic Centers), Countries (United States, Canada, France, Germany, Italy, Spain, United Kingdom, Belgium, Netherlands, Switzerland, China, Japan, India, Australia, South Korea, Thailand, Malaysia, Indonesia, Philippines, Brazil, Mexico, Argentina, South Africa, Saudi Arabia, Turkey, U.A.E., and ROW), Companies (F. Hoffman-La Roche, Eurofins Scientific, Illumina, Natera Inc., Revvity Inc., Thermo Fisher Scientific, Quest Diagnostics, and Agilent Technologies)” provides a detailed analysis of Global Non-invasive Prenatal Testing (NIPT) Market.
Method - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 4 viewpoints:
1. Ultrasound detection2. Biochemical screening tests
3. Cell-free DNA in maternal plasma tests
4. Others
Technology - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 3 viewpoints:
1. ELISA Kits (Enzyme-Linked Immunosorbent Assay Kits)2. Next Generation Sequencing Systems
3. Microarray Analysis
Risk Type - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 2 viewpoints:
1. High & Average risk2. Low risk
End-User - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 3 viewpoints:
1. Specialty Centers2. Hospitals
3. Diagnostic Centers
Countries - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 27 viewpoints:
1. North America
1.1 United States1.2 Canada
2. Europe
2.1 France2.2 Germany
2.3 Italy
2.4 Spain
2.5 United Kingdom
2.6 Belgium
2.7 Netherlands
2.8 Switzerland
2.9 Turkey
3. Asia Pacific
3.1 China3.2 Japan
3.3 India
3.4 Australia
3.5 South Korea
3.6 Thailand
3.7 Malaysia
3.8 Indonesia
3.9 Philippines
4. Latin America
4.1 Brazil4.2 Mexico
4.3 Argentina
5. Middle East & Africa
5.1 South Africa5.2 Saudi Arabia
5.3 UAE
6. Rest of the World
All companies have been covered from 3 viewpoints:
- Overview
- Recent Development
- Revenue
Company Analysis:
1. F. Hoffman-La Roche2. Eurofins Scientific
3. Illumina
4. Natera Inc.
5. Revvity Inc.
6. Thermo Fisher Scientific
7. Quest Diagnostics
8. Agilent Technologies
Table of Contents
Companies Mentioned
- F. Hoffman-La Roche
- Eurofins Scientific
- Illumina
- Natera Inc.
- Revvity Inc.
- Thermo Fisher Scientific
- Quest Diagnostics
- Agilent Technologies
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 210 |
| Published | July 2023 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value ( USD | $ 3.38 Billion |
| Forecasted Market Value ( USD | $ 9.37 Billion |
| Compound Annual Growth Rate | 13.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |