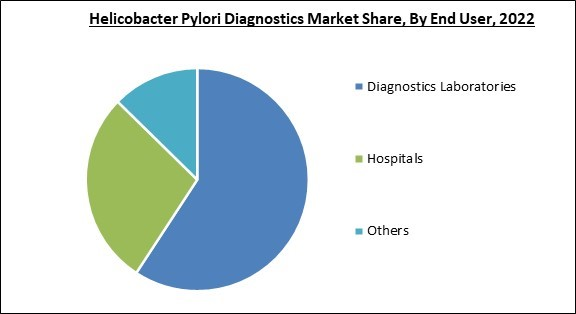
Diagnostic Laboratories are emerging rapidly due to the rising need for diagnosis of various diseases. Therefore, the diagnostic laboratories segment is expected to generate more than 57% share of the market by 2029. H. pylori infection is responsible for about 89% of all cases of gastric cancer, thus, the need for diagnosis emerges. For example, Gastric cancer will cause 770,000 deaths and 1.1 million new cases worldwide 2020. With variations among nations, male and female incidence rates were, on average, two times higher (15.8 and 7.0 per 100,000, respectively). The highest incidence rates were found in Eastern Asia for both males and females (32.5 and 13.2, respectively); the highest rates were found for males living in Japan (48.1), Mongolia (47.2), and Korea (39.7). With incidence rates of under 5 per 100,000, Africa had the lowest incidence. Some of the factors impacting the market are infectious illness prevalence is increasing, creation of novel and enhanced testing techniques, and limitations of diagnostic methods.
In many nations in 2020, taking a flight across international borders is as commonplace as taking a bus or rail to another city. It is incredibly challenging to stop the spread of many diseases since air travel makes it feasible for someone to fly halfway around the world in less time than it takes for many infections to incubate. As a result, the mentioned reasons are raising the incidence of infectious disorders like helicobacter pylori, raising the demand for diagnosis, and supporting market growth. A biosensor is created to pair a transducer and a bioreceptor, which transforms biological activity into a quantifiable signal. Based on the recognition process, the bio-detection systems are categorized into bio-catalytic or bio-affinity-based systems. The need for these diagnosis techniques will rise due to this advancement in diagnosing helicobacter pylori, driving the market's expansion.
However, some organisms (Brucella and H. pylori) split urea quickly, while others react slowly. Thus, for complete identification, it is recommended that biochemical and serological tests be undertaken on pure culture colonies. Inoculum from a bouillon suspension should not be used to promote growth and the urea hydrolysis reaction. Thus, the limitations associated with various diagnostic methods of helicobacter pylori are expected to hinder market growth.
The pandemic has caused non-urgent diagnostic procedures, such as H. pylori tests, to be delayed or abandoned. As a result, there has been a drop in the demand for H. pylori testing, which has impacted the earnings of businesses involved in this market. However, after the pandemic, the market begin to stabilize and show steady growth for the market. The availability of efficient treatment options for H. pylori infection and the development of novel, reasonably priced diagnostic technologies are credited with this. These factors are anticipated to propel the expansion of the H. pylori diagnostics market in the upcoming years.
End-user Outlook
By end user, the market is classified into diagnostics laboratories, hospitals and others. The hospitals segment recorded a significant revenue share in the market in 2022. The segment growth is the result of the accessibility of doctors, the ease with which ailments can be presented, and the growing occurrence of conditions, including small intestinal infections, stomach cancer, gastritis, and duodenal and gastric ulcers. Also, hospitals are known for having cutting-edge technology and staff that guarantee adequate care and diagnosis, which is aiding the market segment to grow in the projected period.Test Type Outlook
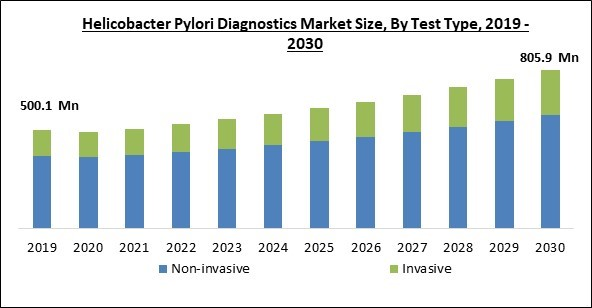
Based on test type, the market is segmented into non-invasive test and invasive test. The non-invasive test segment dominated the market with maximum revenue share in 2022. This is due to its many advantages over invasive tests, the advancement of non-invasive testing methods, and the rise in the incidence of stomach ulcers linked to H. pylori gastritis, non-invasive diagnostics have become more and more popular. Some non-invasive diagnostics, referred to as 'active tests,'can identify an illness that is actively spreading, such as the urea breath test and the stool antigen test. In the primary care context, non-invasive test-and-treat strategies are strongly advised.Method Outlook
On the basis of method, the market is divided into laboratory-based test and point of care test. The laboratory-based test segment witnessed the largest revenue share in the market in 2022. This is owing to their accuracy, usability, and accessibility; laboratory-based tests like the immunoassay have a high adoption rate. To ensure safe shipping, the clinician collects and packages a sample from the patient. This sample is sent to a central lab where a technician will process it. Results from lab tests can typically be generated within 1-2 days, depending on their complexity, and a report is returned to the practitioner.Regional Outlook
Region-wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region led the market by generating the maximum revenue share in 2022. This is because the biggest healthcare spending is in North America, which facilitates easier access to diagnostic tests for H pylori testing. A robust healthcare infrastructure, high purchasing power, and increased usage of helicobacter pylori diagnostic tests are anticipated to fuel market expansion. Additionally, the market is boosted by product approvals, acquisitions, and agreements made by the major regional competitors, providing more growth opportunities for the market.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Cardinal Health, Inc., Biohit Oyj, Abbott Laboratories, Quidel Corporation, Gulf Coast Scientific, Inc. (Gulf Coast Medical Center), Meridian Bioscience, Inc. (SD Biosensor Co., Ltd.), Avanos Medical, Inc., Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc. and F. Hoffmann-La Roche Ltd.
Scope of the Study
By Test Type
- Non-invasive
- Invasive
By End-user
- Diagnostics Laboratories
- Hospitals
- Others
By Method
- Laboratory Based Test
- Point of Care Test
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Cardinal Health, Inc.
- Biohit Oyj
- Abbott Laboratories
- Quidel Corporation
- Gulf Coast Scientific, Inc. (Gulf Coast Medical Center)
- Meridian Bioscience, Inc. (SD Biosensor Co., Ltd.)
- Avanos Medical, Inc.
- Thermo Fisher Scientific, Inc.
- Bio-Rad Laboratories, Inc.
- F.Hoffmann-La Roche Ltd.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Cardinal Health, Inc.
- Biohit Oyj
- Abbott Laboratories
- Quidel Corporation
- Gulf Coast Scientific, Inc. (Gulf Coast Medical Center)
- Meridian Bioscience, Inc. (SD Biosensor Co., Ltd.)
- Avanos Medical, Inc.
- Thermo Fisher Scientific, Inc.
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche Ltd.