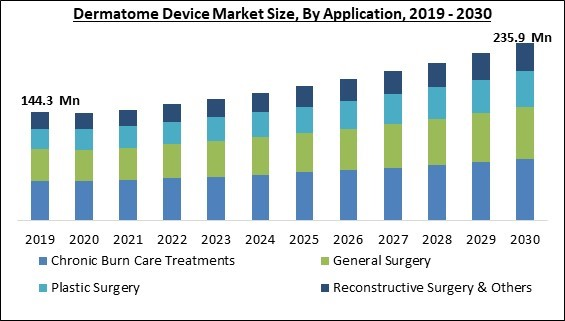
Dermatome Devices are highly utilized in Chronic Burn Care Treatments therefore it is anticipated to register approximately 2/5thshare of the market by 2030. Burns are among the many severe and complex injuries that mass trauma or catastrophes like fires and explosions can produce. According to the World Health Organization's most recent estimates, over a hundred thousand burn-related deaths occur annually around the globe. In the United States alone, direct medical expenses for treating burn victims in children topped US$ 211 million. Over 400,000 Americans undergo treatment each year for burn injuries, according to a research article by the American Burn Association that was released in 2021.
Similarly, it is estimated that US$ 26 million is spent yearly in South Africa to treat burn injuries caused by kerosene (paraffin) cookstove accidents. Therefore, appropriate therapy, such as skin grafting, is needed for burn or fire-related injuries. Rebuilding areas injured by grade 3 burns is one of the main uses for dermatome devices. Some of the factors impacting the market are increasing need for dermatome devices in instances of diabetic foot ulcers, the growing concentration of companies on developing better devices, and risks associated with higher costs for cosmetic surgery.
The rising prevalence of diabetic foot ulcers has been significantly impacting the demand for dermatome devices. The main reason for non-traumatic amputations today is diabetes. The frequency of diabetic foot ulcers worldwide was 6.3%, greater in men than in women, according to a study by the National Center for Biotechnology Information. A large percentage of adults over 60 are affected with chronic leg ulcers. As a result, an increase in the prevalence of diabetic ulcers is predicted to fuel market expansion. Due to important technological advancements, the demand for medical devices for patient care has increased at an unheard-of rate in recent years. Players in the market concentrate primarily on developing advanced powered and air dermatome devices and extending their product portfolios. This will continue to fuel market expansion in the coming years.
However, dermatome devices, as well as skin grafting methods, have rapidly advanced during the past few years. Better control and more accurate, efficient results are probably guaranteed by the interchangeable blades. Due to the increased expense of cosmetic procedures, nations with low GDP per capita are predicted to limit the market growth. Therefore, all these elements can impact the demand and slow market growth. The COVID-19 outbreak resulted in a considerable decrease in the sales of dermatome devices. Lockdowns and stringent government rules to prevent the transmission of the virus, as well as shifts in the priority of medical care, were among the factors that had a detrimental influence on the market. The delay of many elective surgical procedures contributed to decreased overall market revenue.
Product Outlook
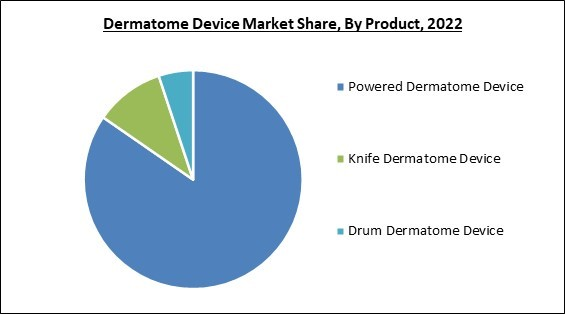
Based on product, the market is characterized into drum dermatome device, knife dermatome device, and powered dermatome device. The drum dermatome device segment procured a considerable growth rate in the market in 2022. Drum dermatomes are generally user-friendly and easy to operate. The devices are designed with ergonomic handles and intuitive controls, making them comfortable to hold and manipulate during the procedure. The simplicity of operation reduces the learning curve for healthcare professionals and improves procedural efficiency. Drum dermatomes are often more affordable compared to other types of dermatome devices.Powdered Outlook
The powered dermatome segment is further classified into pneumatic, battery operated, and electric. The pneumatic segment acquired the largest revenue share in the market in 2022. Pneumatic dermatomes are designed to be user-friendly and easy to operate. They often have intuitive controls and ergonomic designs, allowing healthcare professionals to perform the grafting procedure efficiently and comfortably. The ease of use reduces the learning curve and saves time during surgical procedures. Pneumatic dermatomes offer safety features contributing to patient and operator safety during the procedure.Application Outlook
By application, the market is divided into plastic surgery, reconstructive surgery, general surgery, chronic burn care treatments, and others. The plastic surgery segment garnered a remarkable growth rate in the market in 2022. Plastic surgery is becoming increasingly popular because of factors, including technical developments that have made the treatment safer, shortened the recuperation period, and decreased the cost of the procedure. People are no longer as reluctant to get plastic surgery. Other variables anticipated to promote segment expansion during the projected period include the increasing impact of social media and the rising disposable income.End -Use Outlook
Based on end-use, the market is segmented into hospitals, ambulatory surgical centers, dermatology clinics, and others. The ambulatory surgical centers segment acquired a substantial revenue share in the market in 2022. This is brought on by the growing demand for aesthetic or cosmetic procedures performed in ambulatory surgical centers (ASCs). Additionally, it has benefits, including lower costs by reducing the overall length of the patient's stay following the treatment. As a result, it is the best choice for people who don't require lengthy hospital stays, which is driving up segment demand.Regional Outlook
Region wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment recorded the largest revenue share in the market in 2022. The growth of the market in this region is explained by elements including the presence of well-established market participants, a stable healthcare infrastructure, and a favorable reimbursement environment. Additionally, the nation is now among the top three nations in the world for plastic surgery rates. The majority of plastic surgeons worldwide practice in this nation. As a result, it is projected that increased demand for the product will occur over the projection period.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Zimmer Biomet Holdings, Inc., Integra LifeSciences Holdings Corporation, Aesculap, Inc. (B. Braun Melsungen AG), Surtex Instruments Ltd., Richard Wolf GmbH, Shaanxi Xingmao Industry Co., Ltd., Aygun Surgical Instruments Co., Inc. (CEECAT Capital), Humeca BV (Holland Venture), Nouvag AG and Novo Surgical Inc.
Scope of the Study
By Application
- Chronic Burn Care Treatments
- General Surgery
- Plastic Surgery
- Reconstructive Surgery & Others
By Product
- Powered Dermatome Device
- Pneumatic
- Battery Operated
- Electric
- Knife Dermatome Device
- Drum Dermatome Device
By End-use
- Hospitals
- Ambulatory Surgical Centers
- Dermatology Clinics
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Zimmer Biomet Holdings, Inc.
- Integra LifeSciences Holdings Corporation
- Aesculap, Inc. (B. Braun Melsungen AG)
- Surtex Instruments Ltd.
- Richard Wolf GmbH
- Shaanxi Xingmao Industry Co., Ltd.
- Aygun Surgical Instruments Co., Inc. (CEECAT Capital)
- Humeca BV (Holland Venture)
- Nouvag AG
- Novo Surgical Inc.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Zimmer Biomet Holdings, Inc.
- Integra LifeSciences Holdings Corporation
- Aesculap, Inc. (B. Braun Melsungen AG)
- Surtex Instruments Ltd.
- Richard Wolf GmbH
- Shaanxi Xingmao Industry Co., Ltd.
- Aygun Surgical Instruments Co., Inc. (CEECAT Capital)
- Humeca BV (Holland Venture)
- Nouvag AG
- Novo Surgical Inc.