Wearable Injectors: Introduction
Wearable injectors, also known as wearable infusion devices or patch pumps, are innovative medical devices designed to deliver medication or therapeutic substances through the skin into the body. These devices are typically worn on the body, providing a convenient and portable method of drug administration. They offer several uses and benefits in the healthcare field.The benefits of wearable injectors include:
- Improved Patient Adherence: Wearable injectors enhance patient adherence to medication regimens. The convenience and ease of use encourage patients to adhere to their prescribed treatment plans, leading to better health outcomes
- Precise Drug Delivery: Wearable injectors provide precise and controlled drug delivery, ensuring accurate dosing and minimizing the risk of errors. This promotes therapeutic effectiveness and reduces the potential for under or overdosing
- Reduced Needle Anxiety: For patients who experience needle anxiety or fear, wearable injectors offer a less intimidating method of drug administration. They eliminate the need for frequent needle injections, making the process more tolerable and reducing patient discomfort
- Flexibility and Mobility: Wearable injectors offer increased flexibility and mobility compared to traditional injection methods. Patients can wear the device discreetly under clothing and carry out their daily activities without interruption, allowing for a more active and unrestricted lifestyle
- Monitoring and Connectivity: Some wearable injectors come equipped with monitoring features and connectivity capabilities. These devices can track medication usage, provide reminders for dosing, and transmit data to healthcare professionals for remote monitoring. This enhances patient management and enables healthcare providers to intervene if necessary
Wearable Injectors Market Segmentations
The market can be categorised into type, therapy, end user, and region.Market Breakup by Type
- On-Body Injectors
- Off-Body Injectors
Market Breakup by Therapy
- Oncology
- Autoimmune Disease
- Diabetes
- Cardiovascular Disease
- Others
Market Breakup by End User
- Hospitals
- Diagnostic Centres
- Home Care
- Others
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Wearable Injectors Market Overview
The wearable injectors market has witnessed significant growth and is projected to continue expanding in the coming years. Several factors contribute to the positive market scenario.One of the key drivers of market growth is the increasing prevalence of chronic diseases and the growing need for efficient and convenient drug delivery methods. Conditions such as diabetes, autoimmune diseases, cancer, and hormonal disorders require regular administration of medications or therapeutic substances. Wearable injectors offer a portable and user-friendly solution, enabling patients to self-administer medications at home or on-the-go, reducing the need for frequent visits to healthcare facilities.
Moreover, technological advancements in wearable injectors have played a crucial role in market expansion. Manufacturers are introducing innovative features such as programmable dosing, connectivity capabilities, and integration with digital health platforms. These advancements enhance patient convenience, medication adherence, and real-time monitoring, driving the adoption of wearable injectors in both clinical and home healthcare settings.
Furthermore, the shift towards personalized medicine and targeted therapies has fueled market demand for wearable injectors. These devices allow for precise and controlled drug delivery, enabling tailored treatment regimens based on individual patient needs. Wearable injectors offer the flexibility to deliver medications with varying dosages and infusion rates, supporting personalized therapy plans and optimizing treatment outcomes.
Key Players in the Global wearable injectors Market
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the wearable injectors market are as follows:- Amgen
- Becton
- Dickinson and Company
- Dexcom, Inc
- Enable Injections
- Insulet Corporation
- Sensile Medical
- Tandem Diabetes Care
- Ypsomed
- Johnson & Johnson Services
- F. Hoffmann-La Roche Ltd
- Unilife Corporation
- SteadyMed Therapeutics
- Insulet Corporation
- CeQur SA
Table of Contents
Companies Mentioned
- Amgen
- Becton
- Dickinson and Company
- Dexcom, Inc.
- Enable Injections
- Insulet Corporation
- Sensile Medical
- Tandem Diabetes Care
- Ypsomed
- Johnson & Johnson Services
- F. Hoffmann-La Roche Ltd
- Unilife Corporation
- SteadyMed Therapeutics
- Insulet Corporation
- CeQur SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | July 2023 |
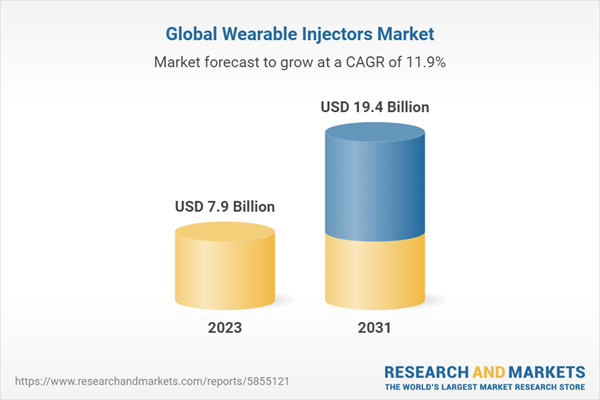
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 7.9 Billion |
| Forecasted Market Value ( USD | $ 19.4 Billion |
| Compound Annual Growth Rate | 11.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |