Minimally Invasive Surgical Devices: Introduction
Minimally invasive surgical devices are devices involved in surgical procedures with minimal incisions and stitches. The term minimally invasive refers to minimal incisions that are made on human body in order to perform a surgery with minimum stitches. These devices do not support a specific definition. The small incisions made by minimally invasive devices enable a patient to recover much faster. There are multiple kind of minimally invasive surgical devices that are used in surgeries such as arthroscopic or laparoscopic devices, and even remote-controlled devices that are manipulated with indirect observation through an endoscope or large display panel by a healthcare professional.Global Minimally Invasive Surgical Devices Market Analysis
The increasing focus on developing more minimally invasive surgical devices backed by advanced technology has been significantly driving the market growth. These advanced technologies might be used in the development of advanced imaging systems, miniature, and flexible devices, along with devices that could also be manipulated from a distance just like remote control devices. The rising competition amongst the key players is leading to the launch of such advanced products in the market, significantly propelling the minimally invasive surgical devices market development.Robot assisted surgeries are already aiding the global minimally invasive surgical devices market growth. The results of these robot assisted surgeries are much more accurate and positive. This feedback is backed by the statements of real healthcare professionals being a part of these robot assisted surgeries. Due to the list of benefits these minimally invasive surgical devices provide, their demand is likely to continue rising.
Global Minimally Invasive Surgical Devices Market Segmentations
Minimally Invasive Surgical Devices Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Handheld Instruments
- Graspers
- Retractors/Elevators
- Dilators
- Suturing Instruments
- Others
- Guiding Devices
- Guiding Catheters
- Guidewires
- Electrosurgical Devices
- Electrosurgery Instruments and Accessories
- Electrosurgery Generators
- Patient Return Electrodes
- Others
- Surgical Scopes
- Endoscope
- Laparoscope
- Gastroscope
- Cystoscope
- Ureteroscope
- Others
- Ablation Devices
- Monitoring and Visualization Devices
- Robotic-assisted Surgical Systems
- Others
Market Breakup by Applications
- Gastrointestinal Surgery
- Gynaecology Surgery
- Urology Surgery
- Orthopaedic and Spine Surgery
- Bariatric Surgery
- Breast Surgery
- Cardiac Surgery
- Cholecystectomy Surgery
- Colectomy Surgery
- Colon and Rectal Surgery
- Ear, Nose, and Throat Surgery
- Others
Market Breakup by End User
- Hospitals Surgical Department
- Outpatient Surgery Patients
- Individual Surgeons
- Group Practice
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Minimally Invasive Surgical Devices Market Overview
The increasing prevalence of chronic diseases are escalating the demand for minimally invasive surgical procedures over traditional surgical procedures, contributing significantly to the growth of the market. The variety of medical specialization and indications that can be treated utilizing these devices are also anticipated to aid the minimally invasive surgical devices market expansion. Minimally invasive surgical devices are being used more frequently in a range of surgical procedures including gastrointestinal, urological, and cardiovascular surgeries. The increased involvement of advanced technologies in order to develop more efficient minimally invasive devices that would provide more accuracy and precision while performing these surgeries. The market is growing steadily and is expected to grow significantly in the coming years.Global Minimally Invasive Surgical Devices Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Stryker
- CONMED Corporation
- Intuitive Surgical
- NuVasive, Inc.
- Fortimedix Surgical
- Microline Surgical
- Cirtec Medical
- OmniGuide Holdings, Inc.
- Arthrex, Inc.
- Eximis Surgical, Inc.
- B. Braun Melsungen, AG
- Medtronic
- Johnson & Johnson Services, Inc.
- Abbott
- Zimmer Biomet
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Stryker
- CONMED Corporation
- Intuitive Surgical
- NuVasive, Inc.
- Fortimedix Surgical
- Microline Surgical
- Cirtec Medical
- OmniGuide Holdings, Inc.
- Arthrex, Inc.
- Eximis Surgical, Inc.
- B. Braun Melsungen, AG
- Medtronic
- Johnson & Johnson Services, Inc.
- Abbott
- Zimmer Biomet
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
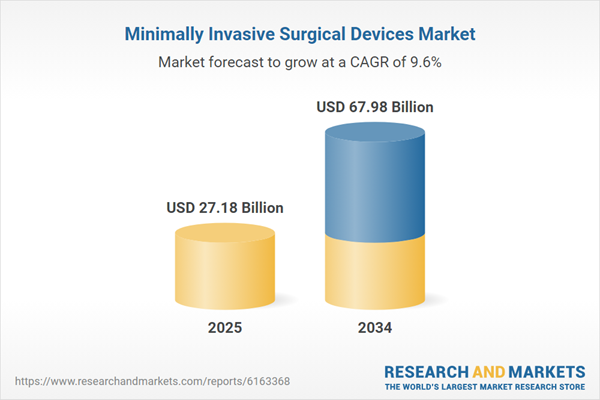
| Estimated Market Value ( USD | $ 27.18 Billion |
| Forecasted Market Value ( USD | $ 67.98 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |