Healthcare Contract Manufacturing: Introduction
Healthcare contract manufacturing, also known as medical contract manufacturing or pharmaceutical contract manufacturing, refers to the outsourcing of the production and manufacturing of medical devices, pharmaceuticals, biotechnology products, and other healthcare-related products to specialized third-party manufacturers. In this arrangement, healthcare companies, including pharmaceutical companies, medical device manufacturers, and biotechnology firms, partner with contract manufacturers to produce their products on their behalf.Key Trends in Healthcare Contract Manufacturing Market
Here are some key trends in the healthcare contract manufacturing market:- Increased Outsourcing in Pharmaceutical Industry: Pharmaceutical companies were increasingly outsourcing manufacturing operations to contract manufacturers. This trend was driven by the desire to focus on research and development, while relying on specialized contract manufacturers to handle the complexities of production and scale-up
- Growing Biopharmaceutical Sector: The biopharmaceutical sector, including biologics and biosimilars, was witnessing significant growth. Contract manufacturing organizations (CMOs) with expertise in biopharmaceutical production were in high demand to meet the increasing global market needs
- Demand for Specialized Services: As the healthcare industry continued to innovate and introduce novel therapies and medical devices, the demand for specialized contract manufacturing services increased. CMOs that offered advanced technology and expertise in specific therapeutic areas or complex formulations gained a competitive edge
- Advancements in Technology: Contract manufacturing organizations were investing in advanced manufacturing technologies and automation to improve efficiency, reduce production costs, and meet higher quality standards
- Focus on Regulatory Compliance: Regulatory compliance remained a critical aspect of healthcare contract manufacturing. CMOs were expected to adhere to stringent regulations imposed by health authorities in various regions to ensure product safety and quality
- Flexible and Agile Manufacturing: Healthcare companies sought contract manufacturers with flexible and agile manufacturing capabilities to respond quickly to changing market demands and efficiently scale up or down production volumes as needed
Healthcare Contract Manufacturing Market Segmentations
The market can be categorised into service, type, manufacturing, end user and region.Market Breakup by Service
Medical Device
- Service
- Accessories Manufacturing
- Assembly Manufacturing
- Component Manufacturing
- Device Manufacturing
- Therapeutic Area
- Cardiology
- Diagnostic Imaging
- Orthopedic
- IVD
- Ophthalmic
- General and Plastic Surgery
- Drug Delivery
- Dental
- Endoscopy
- Diabetes Care
- Others
Pharmaceutical
- Service
- API/Bulk Drugs
- Advanced Drug Delivery Formulation
- Packaging
- Finished Dose Formulations
o Liquid
o Semisolid
Market Breakup by Type
- Sterile
Non-Sterile
- Global Class I
- Class II
- Class III
Market Breakup by Type of Manufacturing
- Raw Material
- Electronics
- Finished Goods
Market Breakup by End User
- Medical Device Companies
- Pharmaceutical Companies
- Biopharmaceutical Companies
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Healthcare Contract Manufacturing Market Overview
The healthcare contract manufacturing market is experiencing robust growth, driven by several key factors that are shaping its expansion and relevance in the healthcare industry. One of the primary drivers is the increasing complexity of healthcare products and therapies, including pharmaceutical drugs and medical devices. As healthcare companies focus on research and development, they turn to contract manufacturers with specialized expertise and advanced technology to handle the intricacies of production, ensuring high-quality and efficient manufacturing processes.Additionally, the rising demand for biopharmaceuticals, including biologics and biosimilars, is fueling the need for contract manufacturing organizations (CMOs) with expertise in biopharmaceutical production. As these therapies gain prominence in treating various diseases, pharmaceutical companies seek contract manufacturers capable of meeting the growing global market demands for biopharmaceutical products.
Moreover, the quest for cost-effective manufacturing solutions is a significant driver in the healthcare contract manufacturing market. By outsourcing production to CMOs, healthcare companies can optimize operational costs, reduce capital investment, and streamline their manufacturing processes. This cost efficiency enables healthcare companies to allocate resources towards other critical areas, such as research, marketing, and distribution.
Regulatory compliance is another crucial driver for healthcare contract manufacturing. CMOs are expected to adhere to stringent regulatory guidelines and maintain high-quality standards imposed by health authorities to ensure product safety and efficacy. Healthcare companies partner with CMOs that can demonstrate a track record of compliance, providing assurance that their products will meet the required regulatory standards.
Moreover, the increasing demand for personalized medicine and precision therapies is driving the need for flexible and agile manufacturing capabilities. Contract manufacturers with the ability to accommodate small-batch productions and individualized treatments are preferred by healthcare companies to cater to the evolving trends in personalized healthcare.
Key Players in the Healthcare Contract Manufacturing Market
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in Healthcare Contract Manufacturing market are as follows:- Thermo Fisher Scientific
- Lonza
- Celestica Inc
- Integer Holdings Corporation
- Nordson Corporation
- Sanmina Corporation
- Phillips-Medisize - a Molex Company
- Catalent, Inc
- Boehringer Ingelheim International GmbH
- Samsung Biologics
- WuXi AppTec
- Cambrex Corporation
- Recifarm
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific
- Lonza
- Celestica Inc.
- Integer Holdings Corporation
- Nordson Corporation
- Sanmina Corporation
- Phillips-Medisize - a Molex Company
- Catalent, Inc
- Boehringer Ingelheim International GmbH
- Samsung Biologics
- WuXi AppTec
- Cambrex Corporation
- Recifarm
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | July 2023 |
| Forecast Period | 2023 - 2031 |
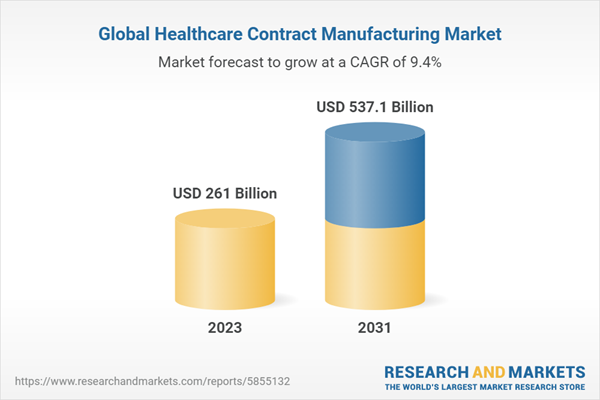
| Estimated Market Value ( USD | $ 261 Billion |
| Forecasted Market Value ( USD | $ 537.1 Billion |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |