Recombinant Cell Culture Supplements: Introduction
Recombinant cell culture supplements play a crucial role in the development and growth of cells in laboratory settings. These supplements provide essential nutrients, growth factors, and other bioactive molecules necessary for optimal cell culture conditions. They are commonly used in various fields such as biopharmaceutical production, regenerative medicine, and research applications.The introduction of recombinant cell culture supplements has revolutionized cell culture techniques by offering enhanced performance, consistency, and reproducibility compared to traditional animal-derived supplements. These products are derived from recombinant DNA technology, where specific genes are inserted into host cells to produce desired proteins or growth factors.
Key Trends in the Recombinant Cell Culture Supplements Market
One of the key trends in the field of recombinant cell culture supplements is the shift towards serum-free and animal component-free formulations. Traditional cell culture supplements often contain serum derived from animal sources, which can introduce variability and potential risks such as contamination and transmission of infectious agents. Serum-free and animal component-free supplements provide a safer and more controlled environment for cell culture, ensuring the reproducibility of results.Overall, the field of recombinant cell culture supplements is witnessing a shift towards serum-free and animal component-free formulations, customization for specific cell types or applications, increased focus on quality control and regulatory compliance, and integration with advanced cell culture technologies. These trends reflect the ongoing efforts to improve cell culture techniques, enhance reproducibility, and meet the evolving needs of various industries relying on cell-based research and production.
Recombinant Cell Culture Supplements Market Segmentations
Market Breakup by Product Type
- Recombinant Albumin (rAlbumin)
- Recombinant Insulin (rInsulin)
- Recombinant Epidermal Growth Factor (rEGF)
- Recombinant Interleukin Growth Factor (rILGF)
- Recombinant Transferrin (rTransferrin)
- Recombinant Trypsin (rTrypsin)
- Recombinant Insulin-like Growth Factor (rIGF)
- Recombinant Stem Cell Factor (rSCF)
- Recombinant Aprotinin (rAprotinin)
- Recombinant Lysozyme (rLysozyme)
Others
- Recombinant Transforming Growth Factor (rTGF)
- Recombinant Insulin-Transferrin-Selenium-Ethanolamine (rITSE)
- Recombinant Platelet-Derived Growth Factor (rPDGF)
- Recombinant Leukemia Inhibitory Factor (rLIF)
- Others
Market Breakup by Application
Regenerative Medicine
- Stem Cell Therapies
- Cell Therapies
- Gene Therapies
Bio-Production
- Monoclonal Antibodies
- Recombinant Proteins
- Hormones
- Vaccines
- Other Biological Products
- Mammalian Expression System
- E. coli Expression System
- Yeast Expression System
- Others
Market Breakup by End User
- Academic and Research Institutions
- Biotechnology and Pharmaceutical Companies
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Recombinant Cell Culture Supplements Market Scenario
The market for recombinant cell culture supplements is experiencing significant growth and is driven by various factors such as the increasing demand for cell-based therapies, advancements in biopharmaceutical production, and the rising focus on precision medicine and personalized therapies. These supplements play a crucial role in providing the necessary components for optimal cell growth, viability, and productivity in various applications.The market is witnessing a shift towards serum-free and animal component-free supplements, driven by the need for safer and more standardized cell culture conditions. This shift is particularly prominent in industries such as biopharmaceuticals, where stringent quality control and regulatory compliance are essential. Serum-free and animal component-free supplements offer improved reproducibility, reduced risk of contamination, and better control over cell culture parameters.
Overall, the market for recombinant cell culture supplements is poised for significant growth, driven by the increasing demand for cell-based therapies, advancements in biopharmaceutical production, customization for specific cell types and applications, adoption of advanced cell culture technologies, and expanding geographical presence.
Recombinant Cell Culture Supplements Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Abcam plc
- Corning Incorporated
- BBI Solutions OEM Limited
- FUJIFILM Irvine Scientific, Inc
- Gemini Bioproducts, LLC
- HiMedia Laboratories, LLC
- Kingfisher Biotech, Inc
- Lonza Group AG
- Merck KGaA
- Novus Biologicals, LLC
- Thermo Fisher Scientific Inc
- InVitria *The publisher always strives to provide you with the latest information. The numbers in the article are only indicative and may be different from the actual report.
Table of Contents
Companies Mentioned
- Abcam plc.
- Corning Incorporated
- BBI Solutions OEM Limited
- FUJIFILM Irvine Scientific, Inc.
- Gemini Bioproducts, LLC
- HiMedia Laboratories, LLC
- Kingfisher Biotech, Inc.
- Lonza Group AG
- Merck KGaA
- Novus Biologicals, LLC
- Thermo Fisher Scientific Inc.
- InVitria
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | July 2023 |
| Forecast Period | 2023 - 2031 |
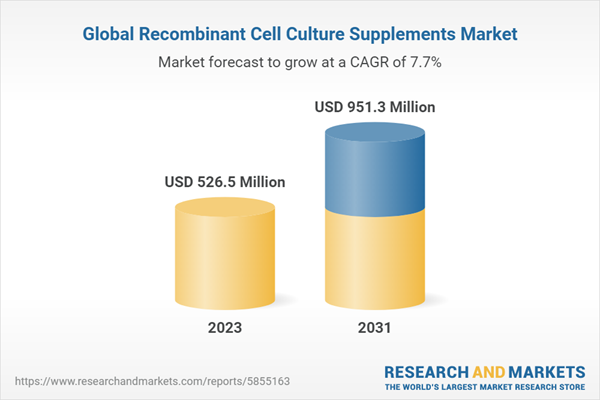
| Estimated Market Value ( USD | $ 526.5 Million |
| Forecasted Market Value ( USD | $ 951.3 Million |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |