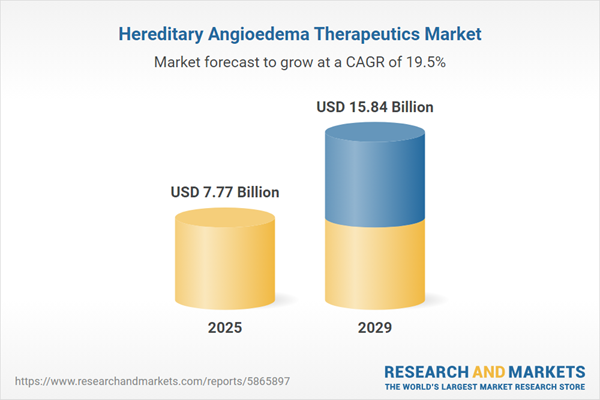
The hereditary angioedema therapeutics market size is expected to see rapid growth in the next few years. It will grow to $15.84 billion in 2029 at a compound annual growth rate (CAGR) of 19.5%. The growth in the forecast period can be attributed to advancements in targeted therapies, global market expansion, gene therapy developments, personalized medicine trends, and collaboration in research and treatment. Major trends in the forecast period include global access initiatives, health economic outcomes research, early intervention strategies, and pediatric HAE therapeutics.
The forecast of 19.5% growth over the next five years reflects a modest reduction of 0.2% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff escalations are likely to burden U.S. rare disease patients by driving up the cost of C1 esterase inhibitor concentrates and bradykinin receptor antagonists imported from Switzerland and Austria, increasing acute attack management costs and emergency care utilization. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The anticipated rise in the prevalence of hereditary angioedema is expected to drive the growth of the hereditary angioedema market in the future. Hereditary angioedema (HAE), a rare genetic disorder characterized by recurrent severe swelling of the skin and mucous membranes, affects approximately 1 in every 50,000 individuals globally, with prevalence estimates ranging from 1,10,000 to 1,150,000. These episodes result in 15,000 to 30,000 emergency room visits annually in the US alone. The increasing incidence of hereditary angioedema is a significant factor propelling the growth of the hereditary angioedema market.
The rising healthcare expenditure is projected to enhance the growth of the hereditary angioedema therapeutics market in the future. Healthcare expenditure refers to the total resources, including financial investments, allocated for healthcare goods and services within a specific timeframe in a defined geographical area. Increased healthcare spending facilitates greater investment in research and development aimed at understanding the underlying causes of hereditary angioedema and creating innovative therapeutic solutions. For instance, in December 2022, the Centers for Medicare & Medicaid Services, a U.S.-based federal agency, reported that U.S. healthcare spending increased by 4.1% to reach $4.5 trillion in 2022, surpassing the 3.2% growth seen in 2021. Therefore, the rise in healthcare expenditure is propelling the growth of the hereditary angioedema therapeutics market.
A notable trend gaining traction in the hereditary angioedema therapeutics market is the emphasis on product innovation. Leading companies in this market are actively involved in the development of groundbreaking products, including ligand-conjugated (LICA) investigational antisense medicine and gene therapy, to reinforce their market position. In a significant development, Intellia Therapeutics, a clinical-stage biotechnology company based in the US, introduced an Investigational New Drug (IND) named NTLA-2002 in March 2023. This innovative therapy, approved by the United States Food and Drug Administration for hereditary angioedema treatment, allows the company's ongoing Phase 1/2 trial to incorporate the US into the global Phase 2 segment. NTLA-2002, a candidate for in vivo genome editing, aims to permanently lower plasma kallikrein protein activity and prevent hereditary angioedema attacks after a single dose of therapy through the inactivation of the target gene, kallikrein B1 (KLKB1).
Key companies in the hereditary angioedema (HAE) therapeutics market are focusing on developing innovative treatments, such as oral plasma kallikrein inhibitors, to improve patient management and provide effective on-demand solutions for alleviating HAE attacks. These oral medications work by inhibiting the enzyme plasma kallikrein, which helps manage and lessen the severity of hereditary angioedema episodes. For example, in September 2024, KalVista Pharmaceuticals, a U.S.-based biopharmaceutical company, received a new drug application approval from the U.S. Food and Drug Administration for Sebetralstat. This treatment aims to provide HAE patients with a more convenient, non-invasive alternative to intravenous or subcutaneous therapies. It addresses the increasing demand for rapid and effective oral treatments that can be administered immediately during an HAE attack, thereby enhancing overall patient outcomes and quality of life.
In February 2023, Takeda Pharmaceutical Company acquired Nimbus Lakshmi, Inc. from Nimbus Therapeutics, LLC. The acquisition, involving an undisclosed amount, enhances Takeda's late-stage pipeline, contributing to portfolio expansion and broadening its impact across multiple indications. Nimbus Lakshmi is a US-based company specializing in Tyrosine kinase 2 (TYK2) inhibitor NDI-034858.
Major companies operating in the hereditary angioedema therapeutics market include Sanofi S.A., Pharming Healthcare Inc., Attune Pharmaceuticals Inc., Adverum Biotechnologies Inc., Arrowhead Pharmaceuticals Inc., Ionis Pharmaceuticals Inc., BioCryst Pharmaceuticals Inc., CSL Behring LLC, KalVista Pharmaceutical Inc., CENTOGENE N.V., GlaxoSmithKline plc., Eli Lilly and Company, Amgen Inc., AbbVie Inc., Incyte Corporation, BioMarin Pharmaceutical Inc., Dyax Corp., Salix Pharmaceuticals Inc., Pharvaris N.V., Innovent Biologics Inc., AstraZeneca plc, BeiGene Ltd., Jiangsu HenGrui Medicine Co. Ltd., Bristol-Myers Squibb Company, F. Hoffmann-La Roche AG ., Exelixis Inc., Lev Pharmaceuticals Inc., Kedrion Biopharma Inc., Pharos Pharmaceuticals Inc.
North America was the largest region in the global hereditary angioedema therapeutics market in 2024. Asia-pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the hereditary angioedema therapeutics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the hereditary angioedema therapeutics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The hereditary angioedema therapeutics market research report is one of a series of new reports that provides hereditary angioedema therapeutics market statistics, including hereditary angioedema therapeutics industry global market size, regional shares, competitors with a hereditary angioedema therapeutics market share, detailed hereditary angioedema therapeutics market segments, market trends, and opportunities, and any further data you may need to thrive in the hereditary angioedema therapeutics industry. This hereditary angioedema therapeutics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Hereditary angioedema (HAE) therapeutics pertain to the treatment of an autosomal dominant condition caused by a deficiency or malfunction of the C1-inhibitor protein. Symptoms of hereditary angioedema include angioedema-related upper airway, cutaneous, and/or gastrointestinal complaints.
The main drug classes of hereditary angioedema therapeutics include C1 esterase inhibitor, selective bradykinin B2 receptor antagonist, kallikrein inhibitor, and others. C1 Esterase Inhibitor is a protein present in the fluid portion of blood that regulates the complement system's protein C1. These therapeutics are administered through intravenous, subcutaneous, and oral routes and are distributed via hospital pharmacies, retail pharmacies, and others. The application of hereditary angioedema therapeutics includes prophylaxis and on-demand treatment.
The hereditary angioedema therapeutics market includes revenues earned by entities by diagnosis and evaluation, treatment, management of hereditary angioedema. The market value includes the value of related goods sold by the service provider or included within the service offering. he hereditary angioedema therapeutics market also includes of sales of tranexamic acid, danazol, intravenous and subcutaneous nano filtered purified plasma-derived human C1 esterase inhibitor concentrate, and lanadelumab. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Hereditary Angioedema Therapeutics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on hereditary angioedema therapeutics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for hereditary angioedema therapeutics? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The hereditary angioedema therapeutics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Drug Class: C1 Esterase Inhibitor; Selective Bradykinin B2 Receptor Antagonist; Kallikrein Inhibitor; Other Drug Classes2) By Route of Administration: Intravenous; Subcutaneous; Oral
3) By Distribution Channel: Hospital Pharmacy; Retail Pharmacy; Other Distribution Channels
4) By Application: Prophylaxis; on-demand
Subsegments:
1) By C1 Esterase Inhibitor: Plasma-Derived; Recombinant2) By Selective Bradykinin B2 Receptor Antagonist: Icatibant
3) By Kallikrein Inhibitor: Ecallantide; Lanadelumab
4) By Other Drug Classes: Emerging Therapies
Companies Mentioned: Sanofi S.A.; Pharming Healthcare Inc.; Attune Pharmaceuticals Inc.; Adverum Biotechnologies Inc.; Arrowhead Pharmaceuticals Inc.; Ionis Pharmaceuticals Inc.; BioCryst Pharmaceuticals Inc.; CSL Behring LLC; KalVista Pharmaceutical Inc.; CENTOGENE N.V.; GlaxoSmithKline plc.; Eli Lilly and Company; Amgen Inc.; AbbVie Inc.; Incyte Corporation; BioMarin Pharmaceutical Inc.; Dyax Corp.; Salix Pharmaceuticals Inc.; Pharvaris N.V.; Innovent Biologics Inc.; AstraZeneca plc; BeiGene Ltd.; Jiangsu HenGrui Medicine Co. Ltd.; Bristol-Myers Squibb Company; F. Hoffmann-La Roche AG.; Exelixis Inc.; Lev Pharmaceuticals Inc.; Kedrion Biopharma Inc.; Pharos Pharmaceuticals Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Hereditary Angioedema Therapeutics market report include:- Sanofi S.A.
- Pharming Healthcare Inc.
- Attune Pharmaceuticals Inc.
- Adverum Biotechnologies Inc.
- Arrowhead Pharmaceuticals Inc.
- Ionis Pharmaceuticals Inc.
- BioCryst Pharmaceuticals Inc.
- CSL Behring LLC
- KalVista Pharmaceutical Inc.
- CENTOGENE N.V.
- GlaxoSmithKline plc.
- Eli Lilly and Company
- Amgen Inc.
- AbbVie Inc.
- Incyte Corporation
- BioMarin Pharmaceutical Inc.
- Dyax Corp.
- Salix Pharmaceuticals Inc.
- Pharvaris N.V.
- Innovent Biologics Inc.
- AstraZeneca plc
- BeiGene Ltd.
- Jiangsu HenGrui Medicine Co. Ltd.
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche AG.
- Exelixis Inc.
- Lev Pharmaceuticals Inc.
- Kedrion Biopharma Inc.
- Pharos Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 7.77 Billion |
| Forecasted Market Value ( USD | $ 15.84 Billion |
| Compound Annual Growth Rate | 19.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |