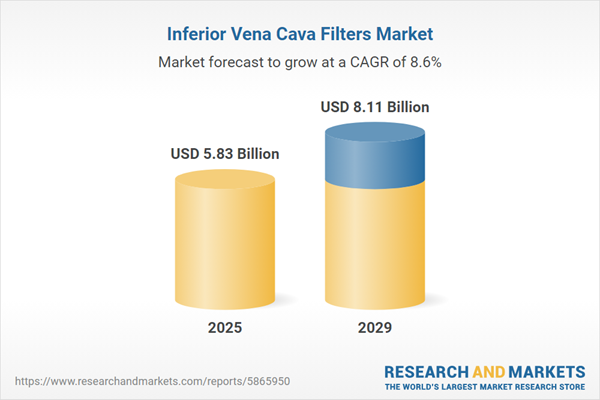
The inferior vena cava filters market size is expected to see strong growth in the next few years. It will grow to $8.11 billion in 2029 at a compound annual growth rate (CAGR) of 8.6%. The growth in the forecast period can be attributed to increasing surgical procedures and trauma cases, focus on outpatient settings, rise in chronic diseases and comorbidities, global aging population trends, expanded access to healthcare services. Major trends in the forecast period include enhanced thrombus capture capabilities, exploration of alternative anticoagulant therapies, increased collaboration between manufacturers and healthcare providers, continuous regulatory compliance and monitoring, growing awareness and education initiatives.
The projected increase in the prevalence of venous thromboembolism is set to drive the growth of the inferior vena cava filter market. Venous thromboembolism (VTE), characterized by blood clots in the veins with potential severe consequences, underscores the importance of inferior vena cava filters. These medical devices are instrumental in preventing blood clots from reaching critical organs such as the heart or lungs. According to data from the Centers for Disease Control and Prevention in February 2023, an estimated 900,000 individuals could be affected by venous thromboembolism, contributing to 60,000 to 100,000 annual deaths in the United States. Hence, the rise in VTE prevalence is a significant factor propelling the growth of the inferior vena cava filter market.
The growing preference for non-invasive or minimally invasive medical procedures is anticipated to be a key driver for the inferior vena cava filter market. Minimally invasive procedures, which aim to minimize disruption to normal bodily structures, often involve the use of inferior vena cava (IVC) filters to manage and prevent complications associated with conditions such as deep vein thrombosis (DVT) and pulmonary embolism (PE). The American Society of Plastic Surgeons reported in September 2023 that approximately 23.7 million cosmetic minimally invasive procedures were performed in 2022, with a significant surge of 73% observed in Neuromodulator Injections compared to 2019. This trend reflects a growing preference for procedures with reduced invasiveness, thereby contributing to the growth of the inferior vena cava filter market.
The increase in surgical procedures is expected to drive the growth of the inferior vena cava (IVC) filters market in the future. Surgical procedures are medical operations conducted to diagnose, treat, and prevent health issues through physical alterations to tissues and organs. This rise in surgical procedures can be largely attributed to technological advancements in medicine, an expanding elderly population, and a growing prevalence of chronic health conditions. The uptick in surgical operations boosts the IVC filters market by increasing the demand for these devices, which play a crucial role in preventing blood clots during and after surgeries. For example, in September 2023, data from the American Society of Plastic Surgeons (ASPS), a U.S.-based non-profit organization, estimated that the total number of cosmetic surgery procedures rose to 1,575,244 in 2023, representing a 5% increase from 1,498,361 procedures in 2022. Consequently, the rise in surgical procedures is fueling the growth of the inferior vena cava (IVC) filters market.
Leading companies in the inferior vena cava (IVC) filters market are developing advanced IVC filters to enhance patient outcomes and improve filter retrieval rates. IVC filters play a vital role by preventing blood clots from reaching the lungs, thereby reducing the risk of pulmonary embolism in patients who are unable to use blood-thinning medications. For example, in April 2022, Royal Philips, a Netherlands-based health technology company, introduced the CavaClear IVC filter removal laser sheath, providing a minimally invasive solution for safely retrieving embedded IVC filters. By utilizing advanced circumferential tissue ablation, this innovation improves procedural efficiency and decreases the number of retrieval attempts, addressing a significant need in the IVC filter market.
Strategic partnerships have become a prevalent approach for major companies in the inferior vena cava filter market, aiming to extend distribution networks and gain a competitive advantage. These partnerships allow companies to broaden their service offerings, expand market reach, and enhance customer satisfaction through synergies. An illustration of this strategy is the investment by Cordis-X, a manufacturer of interventional cardiovascular technologies, in Adient Medical in June 2022. This $11 million investment is intended to diversify Cordis's product portfolio to address unmet needs in cardiovascular health.
Major companies operating in the inferior vena cava filters market include B. Braun Melsungen AG, Argon Medical Devices Inc., Boston Scientific Corporation, Cook Medical LLC, Braile Biomédica Indústria Comércio e Representações Ltd., Lifetech Scientific Corporation, VENITI Inc., Cordis Corporation, Bard, Becton Dickinson and Company, Cardinal Health Inc., C. R. Bard Inc., LeMaitre Vascular Inc., Medtronic plc, Merit Medical Systems Inc., Teleflex Incorporated, RA Medical Systems, ETEX Corporation, OptiMed Medizinische Instrumente GmbH, Blue Sail Medical Co. Ltd., Penumbra Inc.
North America was the largest region in the inferior vena cava filters market in 2024. The regions covered in the inferior vena cava filters market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the inferior vena cava filters market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
An inferior vena cava filter is a small device designed to prevent blood clots from reaching the lungs through the inferior vena cava, a large vein in the body. The inferior vena cava is responsible for returning deoxygenated blood to the heart and lungs. The inferior vena cava filter acts as a barrier, capturing any blood clots that may pass through and preventing them from reaching the heart or lungs.
The main product types of inferior vena cava filters include retrievable inferior vena cava (IVC) filters and permanent inferior vena cava (IVC) filters. Retrievable IVC filters allow percutaneous removal when the risk of blood clot resolves. These filters are made from various materials, including non-ferromagnetic and ferromagnetic materials. They are used in various applications such as the treatment of venous thromboembolism (VTE), prevention of pulmonary embolism (PE), and other relevant medical uses. The devices are typically employed by hospitals, ambulatory surgical centers, and other healthcare facilities.
The inferior vena cava filters market research report is one of a series of new reports that provides inferior vena cava filters market statistics, including inferior vena cava filters industry global market size, regional shares, competitors with an inferior vena cava filters market share, detailed inferior vena cava filters market segments, market trends, and opportunities, and any further data you may need to thrive in the inferior vena cava filter industry. This inferior vena cava filters market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The inferior vena cava filters market consists of sales of resorbable IVC filters, Polydioxanone (PPDO) IVC filters. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Inferior Vena Cava Filters Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on inferior vena cava filters market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for inferior vena cava filters? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The inferior vena cava filters market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Retrievable Inferior Vena Cava (IVC) Filters; Permanent Inferior Vena Cava (IVC) Filters2) By Material: Non-Ferromagnetic Materials; Ferromagnetic Materials
3) By Application: Treatment of Venous Thromboembolism (VTE); Prevention of Pulmonary Embolism (PE); Other Applications
4) By End User: Hospitals; Ambulatory Surgical Centers; Other End Users
Subsegments:
1) By Retrievable IVC Filters: Optional Retrievable Filters; Temporary Filters2) By Permanent IVC Filters: Stainless Steel Filters; Titanium Filters; Other Permanent Filters
Key Companies Mentioned: B. Braun Melsungen AG; Argon Medical Devices Inc.; Boston Scientific Corporation; Cook Medical LLC; Braile Biomédica Indústria Comércio e Representações Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- B. Braun Melsungen AG
- Argon Medical Devices Inc.
- Boston Scientific Corporation
- Cook Medical LLC
- Braile Biomédica Indústria Comércio e Representações Ltd.
- Lifetech Scientific Corporation
- VENITI Inc.
- Cordis Corporation
- Bard
- Becton Dickinson and Company
- Cardinal Health Inc.
- C. R. Bard Inc.
- LeMaitre Vascular Inc.
- Medtronic plc
- Merit Medical Systems Inc.
- Teleflex Incorporated
- RA Medical Systems
- ETEX Corporation
- OptiMed Medizinische Instrumente GmbH
- Blue Sail Medical Co. Ltd.
- Penumbra Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 5.83 Billion |
| Forecasted Market Value ( USD | $ 8.11 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |