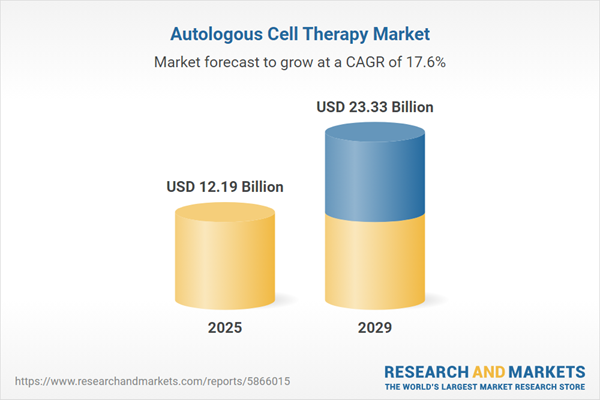
The autologous cell therapy market size is expected to see rapid growth in the next few years. It will grow to $23.33 billion in 2029 at a compound annual growth rate (CAGR) of 17.6%. The growth in the forecast period can be attributed to continued growth in regenerative medicine, expanding applications across medical specialties, advancements in cell culture techniques, increasing investment and research initiatives, regulatory support, and standardization. Major trends in the forecast period include the development of automated manufacturing technologies, integration of biomarkers in treatment strategies, the emergence of point-of-care cell processing, expansion beyond hematopoietic stem cells, collaborations, and partnerships in the industry.
The burgeoning prevalence of chronic diseases is poised to fuel the expansion of autologous cell therapy. Chronic diseases, persisting for more than a year, necessitate continual medical attention. Autologous cell therapy, leveraging an individual's cultivated and amplified cells reintroduced into the donor, holds promise in managing and treating such conditions. According to the National Library of Medicine's January 2023 report, the US is projected to witness 142.66 million people aged 50 and above with at least one chronic condition by 2050. Hence, the heightened prevalence of chronic diseases propels the growth of the autologous cell therapy market.
The increasing aging population is expected to drive the growth of the autologous cell therapy market in the coming years. An aging population refers to the rise in median age within a population due to declining fertility rates and longer life expectancy. Autologous cell therapies can benefit this demographic by providing targeted and personalized treatment options. For instance, in July 2024, the Office of National Statistics, a UK-based producer of official statistics, reported that in 2022, approximately 12.7 million people in the UK were aged 65 and over, representing 19% of the total population. Projections indicate that by 2072, this figure could rise to 22.1 million, making up 27% of the population. Consequently, the growing aging population is fueling the expansion of the autologous cell therapy market.
Technological advancements emerge as a prominent trend shaping the autologous cell therapy market. Market leaders are automating the process to enhance efficiency and ensure sustainability. Lonza, for instance, unveiled the Cocoon Platform in April 2022, a cutting-edge technology streamlining personalized cell and gene therapy manufacturing. This platform, driven by an automated closed system, minimizes contamination risk, upholds manufacturing sterility, substantially bolsters quality, reduces labor and material expenses, and facilitates the scalable production of autologous T-cell products.
Major players in the autologous cell therapy realm are forging strategic partnerships and collaborations to fortify their market positions. Strategic partnerships, fostering collaborative endeavors between multiple entities, aim to achieve specific business objectives. Notably, in March 2022, Novartis inked an initial agreement with Carisma Therapeutics, aiming to manufacture HER 2 targeted CAR-M cell therapy. The collaboration involves transferring Carisma Therapeutics' manufacturing process to Novartis' Cell Therapy Site in the US, scheduling clinical manufacturing to commence in 2023, focusing on treating solid tumors in initial trials.
In October 2023, Clade Therapeutics, a US-based cell therapy company, successfully acquired Gadeta B.V. for an undisclosed sum. This strategic acquisition significantly enriches Clade's repertoire by incorporating innovative gamma/delta (g/d) T-cell receptor (TCR)-based immunotherapies tailored for cancer patients into its existing portfolio. Gadeta B.V., based in the Netherlands, specializes in the development of cell therapies.
Major companies operating in the autologous cell therapy market include Bristol-Myers Squibb Company, Novartis AG, Vericel Corporation, Holostem Terapie Avanzate S.r.l., Pharmicell Co. Ltd., Opexa Therapeutics Inc., Tego Science AB, Brainstorm Cell Therapeutics Inc., Caladrius Biosciences Inc., Lineage Cell Therapeutics Inc., Castle Creek Biosciences Inc., Gilead Sciences Inc., Johnson & Johnson, CORESTEM Inc., Dendreon Pharmaceuticals LLC, Medipost Co. Ltd., PharmaJet Inc., Bioject Medical Technologies Inc., Medical International Technology Inc., INJEX Pharma AG, Lonza Group Ltd., Corning Incorporated, Takeda Pharmaceutical Company Limited, CSL Limited, AstraZeneca plc, Moderna Inc., Sinovac Biotech Ltd., Valneva SE, Bavarian Nordic A/S, Dynavax Technologies Corporation.
North America was the largest region in the autologous cell therapy market in 2024. Asia-pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the autologous cell therapy market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the autologous cell therapy market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Autologous cell therapy stands as a method within cell therapy where an individual's cells are gathered, processed, and subsequently reintroduced into their own body to address therapeutic needs. It plays a vital role in regenerative medicine, aiming to repair, replace, or rejuvenate damaged tissues or organs.
Within autologous cell therapy, two primary types are prevalent, autologous stem cell therapy and autologous cellular immunotherapies. Autologous stem cell therapy, a specific facet of this therapy, harnesses stem cells sourced from the patient's own body for therapeutic purposes. These stem cells are derived from various sources such as bone marrow, epidermis, mesenchymal stem cells, hematopoietic stem cells, chondrocytes, among others. Their applications span across a wide spectrum of medical areas, including cancer, neurodegenerative disorders, cardiovascular issues, autoimmune conditions, orthopedics, and wound healing. This therapy finds application across multiple sectors including hospitals, clinics, ambulatory centers, academic and research institutions, and other healthcare settings, serving as a versatile approach in addressing various health challenges.
The autologous cell therapy market research report is one of a series of new reports that provides autologous cell therapy market statistics, including autologous cell therapy industry global market size, regional shares, competitors with a autologous cell therapy market share, detailed autologous cell therapy market segments, market trends and opportunities, and any further data you may need to thrive in the autologous cell therapy industry. This autologous cell therapy market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The autologous cell therapy market includes revenues earned by providing services in relation to cell collection, cell processing, cell expansion, or its modification. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Autologous Cell Therapy Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on autologous cell therapy market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for autologous cell therapy? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The autologous cell therapy market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Therapy: Autologous Stem Cell Therapy; Autologous Cellular Immunotherapies2) By Source: Bone Marrow; Epidermis; Mesenchymal Stem Cells; Hematopoietic Stem Cells; Chondrocytes; Other Sources
3) By Application: Cancer; Neurodegenerative Disorders; Cardiovascular Disorders; Autoimmune Disorders; Orthopedics; Wound Healing; Other Applications
4) By End User: Hospitals and Clinics; Ambulatory Centers; Academics and Research; Other End-Users
Subsegments:
1) By Autologous Stem Cell Therapy: Hematopoietic Stem Cell Transplantation; Mesenchymal Stem Cell Therapy; Adipose-Derived Stem Cell Therapy2) By Autologous Cellular Immunotherapies: Chimeric Antigen Receptor (CAR) T-Cell Therapy; Tumor-Infiltrating Lymphocyte (TIL) Therapy; Dendritic Cell Therapy
Key Companies Mentioned: Bristol-Myers Squibb Company; Novartis AG; Vericel Corporation; Holostem Terapie Avanzate S.r.l.; Pharmicell Co. Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Bristol-Myers Squibb Company
- Novartis AG
- Vericel Corporation
- Holostem Terapie Avanzate S.r.l.
- Pharmicell Co. Ltd.
- Opexa Therapeutics Inc.
- Tego Science AB
- Brainstorm Cell Therapeutics Inc.
- Caladrius Biosciences Inc.
- Lineage Cell Therapeutics Inc.
- Castle Creek Biosciences Inc.
- Gilead Sciences Inc.
- Johnson & Johnson
- CORESTEM Inc.
- Dendreon Pharmaceuticals LLC
- Medipost Co. Ltd.
- PharmaJet Inc.
- Bioject Medical Technologies Inc.
- Medical International Technology Inc.
- INJEX Pharma AG
- Lonza Group Ltd.
- Corning Incorporated
- Takeda Pharmaceutical Company Limited
- CSL Limited
- AstraZeneca plc
- Moderna Inc.
- Sinovac Biotech Ltd.
- Valneva SE
- Bavarian Nordic A/S
- Dynavax Technologies Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 12.19 Billion |
| Forecasted Market Value ( USD | $ 23.33 Billion |
| Compound Annual Growth Rate | 17.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |