1. Research Methodology
1.1. Study Objectives
1.2. Study Scope
1.3. Research Assumptions
1.4. Research Framework
2. Introduction
2.1. Market Definition
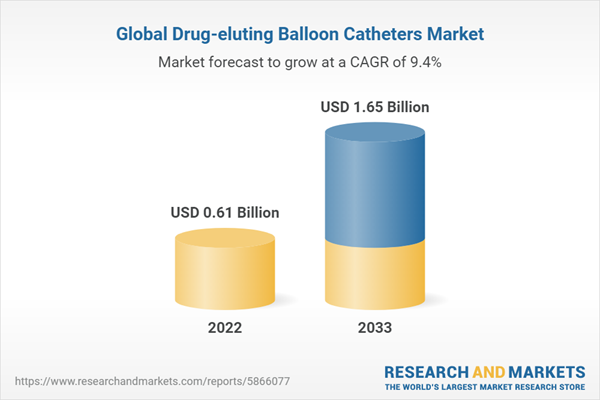
2.2. Global Drug-eluting Balloon Catheters Market Overview
4. Market Environment Analysis
4.1. Porter’s 5 Forces Analysis
4.2. PESTEL Analysis
4.3. SWOT Analysis
5. Market Dynamics
5.1. Drivers Analysis
5.2. Restraints Analysis
5.3. Opportunities Analysis
5.4. Threats Analysis
5.5. Trend Analysis
7. Drug-eluting Balloon Catheters Market: Drug Estimates & Trend Analysis
7.1. Drug Segment Opportunity Analysis
7.2. Sirolimus
7.2.1. Sirolimus Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
7.3. Paclitaxel
7.3.1. Paclitaxel Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
7.4. Others
7.4.1. Others Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
8. Drug-eluting Balloon Catheters Market: Indication Estimates & Trend Analysis
8.1. Indication Segment Opportunity Analysis
8.2. Peripheral Intervention
8.2.1. Peripheral Intervention Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
8.3. Coronary Intervention
8.3.1. Coronary Intervention Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
9. Drug-eluting Balloon Catheters Market: End-user Estimates & Trend Analysis
9.1. End-user Segment Opportunity Analysis
9.2. Specialty Clinics & Catheterization Laboratories
9.2.1. Specialty Clinics & Catheterization Laboratories Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
9.3. Ambulatory Surgical Centers & Hospitals
9.3.1. Ambulatory Surgical Centers & Hospitals Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
9.4. Others
9.4.1. Others Market Analysis & Forecast, 2022-2033 (Revenue, USD Mn)
10. Regional Market Analysis
10.1. Regional Market Opportunity Analysis
11. North America Drug-eluting Balloon Catheters Market
11.1. North America Drug-eluting Balloon Catheters Market
11.1.1. North America Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
11.1.2. North America Drug-eluting Balloon Catheters Market Size and Forecast, By Country, 2022-2033 (Revenue USD Mn)
11.1.3. North America Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
11.1.4. North America Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
11.1.5. North America Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
11.2. U.S. Global Drug-eluting Balloon Catheters Market
11.2.1. U.S. Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
11.2.2. U.S. Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
11.2.3. U.S. Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
11.2.4. U.S. Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
11.3. Canada Global Drug-eluting Balloon Catheters Market
11.3.1. Canada Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
11.3.2. Canada Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
11.3.3. Canada Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
11.3.4. Canada Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
12. Europe Global Drug-eluting Balloon Catheters Market
12.1. Europe Global Drug-eluting Balloon Catheters Market
12.1.1. Europe Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
12.1.2. Europe Drug-eluting Balloon Catheters Market Size and Forecast, By Country, 2022-2033 (Revenue USD Mn)
12.1.3. Europe Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
12.1.4. Europe Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
12.1.5. Europe Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
12.2. Germany Global Drug-eluting Balloon Catheters Market
12.2.1. Germany Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
12.2.2. Germany Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
12.2.3. Germany Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
12.2.4. Germany Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
12.3. UK Global Drug-eluting Balloon Catheters Market
12.3.1. UK Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
12.3.2. UK Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
12.3.3. UK Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
12.3.4. UK Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
12.4. France Global Drug-eluting Balloon Catheters Market
12.4.1. France Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
12.4.2. France Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
12.4.3. France Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
12.4.4. France Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
12.5. Spain Global Drug-eluting Balloon Catheters Market
12.5.1. Spain Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
12.5.2. Spain Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
12.5.3. Spain Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
12.5.4. Spain Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
12.6. Italy Global Drug-eluting Balloon Catheters Market
12.6.1. Italy Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
12.6.2. Italy Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
12.6.3. Italy Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
12.6.4. Italy Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
12.7. Rest of Europe Global Drug-eluting Balloon Catheters Market
12.7.1. Rest of Europe Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
12.7.2. Rest of Europe Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
12.7.3. Rest of Europe Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
12.7.4. Rest of Europe Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
13. Asia Pacific Global Drug-eluting Balloon Catheters Market
13.1. Asia Pacific Global Drug-eluting Balloon Catheters Market
13.1.1. Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
13.1.2. Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, By Country, 2022-2033 (Revenue USD Mn)
13.1.3. Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
13.1.4. Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
13.1.5. Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
13.2. Japan Global Drug-eluting Balloon Catheters Market
13.2.1. Japan Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
13.2.2. Japan Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
13.2.3. Japan Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
13.2.4. Japan Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
13.3. China Global Drug-eluting Balloon Catheters Market
13.3.1. China Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
13.3.2. China Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
13.3.3. China Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
13.3.4. China Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
13.4. India Global Drug-eluting Balloon Catheters Market
13.4.1. India Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
13.4.2. India Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
13.4.3. India Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
13.4.4. India Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
13.5. South Korea Global Drug-eluting Balloon Catheters Market
13.5.1. South Korea Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
13.5.2. South Korea Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
13.5.3. South Korea Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
13.5.4. South Korea Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
13.6. Australia Global Drug-eluting Balloon Catheters Market
13.6.1. Australia Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
13.6.2. Australia Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
13.6.3. Australia Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
13.6.4. Australia Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
13.7. Rest of Asia Pacific Global Drug-eluting Balloon Catheters Market
13.7.1. Rest of Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
13.7.2. Rest of Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
13.7.3. Rest of Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
13.7.4. Rest of Asia Pacific Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
14. Latin America Global Drug-eluting Balloon Catheters Market
14.1. Latin America Global Drug-eluting Balloon Catheters Market
14.1.1. Latin America Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
14.1.2. Latin America Drug-eluting Balloon Catheters Market Size and Forecast, By Country, 2022-2033 (Revenue USD Mn)
14.1.3. Latin America Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
14.1.4. Latin America Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
14.1.5. Latin America Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
14.2. Brazil Global Drug-eluting Balloon Catheters Market
14.2.1. Brazil Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
14.2.2. Brazil Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
14.2.3. Brazil Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
14.2.4. Brazil Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
14.3. Mexico Global Drug-eluting Balloon Catheters Market
14.3.1. Mexico Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
14.3.2. Mexico Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
14.3.3. Mexico Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
14.3.4. Mexico Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
14.4. Argentina Global Drug-eluting Balloon Catheters Market
14.4.1. Argentina Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
14.4.2. Argentina Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
14.4.3. Argentina Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
14.4.4. Argentina Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
14.5. Rest of Latin America Global Drug-eluting Balloon Catheters Market
14.5.1. Rest of Latin America Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
14.5.2. Rest of Latin America Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
14.5.3. Rest of Latin America Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
14.5.4. Rest of Latin America Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
15. MEA Global Drug-eluting Balloon Catheters Market
15.1. MEA Global Drug-eluting Balloon Catheters Market
15.1.1. MEA Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
15.1.2. MEA Drug-eluting Balloon Catheters Market Size and Forecast, By Country, 2022-2033 (Revenue USD Mn)
15.1.3. MEA Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
15.1.4. MEA Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
15.1.5. MEA Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
15.2. GCC Global Drug-eluting Balloon Catheters Market
15.2.1. GCC Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
15.2.2. GCC Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
15.2.3. GCC Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
15.2.4. GCC Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
15.3. South Africa Global Drug-eluting Balloon Catheters Market
15.3.1. South Africa Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
15.3.2. South Africa Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
15.3.3. South Africa Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
15.3.4. South Africa Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
15.4. Rest of MEA Global Drug-eluting Balloon Catheters Market
15.4.1. Rest of MEA Drug-eluting Balloon Catheters Market Size and Forecast, 2022-2033 (Revenue USD Mn)
15.4.2. Rest of MEA Drug-eluting Balloon Catheters Market Size and Forecast, By Drug, 2022-2033 (Revenue USD Mn)
15.4.3. Rest of MEA Drug-eluting Balloon Catheters Market Size and Forecast, By Indication, 2022-2033 (Revenue USD Mn)
15.4.4. Rest of MEA Drug-eluting Balloon Catheters Market Size and Forecast, By End-user, 2022-2033 (Revenue USD Mn)
16. Competitor Analysis
16.1. Company Market Share Analysis, 2022
16.2. Major Recent Developments
17. Company Profiles
17.1. MedAlliance
17.2. MicroPort Medical Co. Ltd.
17.3. Koninklijke Philips N.V.
17.4. BD
17.5. Boston Scientific Corporation
17.6. Medtronic
17.7. B. Braun SE
17.8. Merial Life Sciences Pvt. Ltd.
17.9. Cordis
17.10. Advanced NanoTherapies
17.11. BIOTRONIK
17.12. Other Prominent Players