The steep rise in per-capita healthcare spending, in addition to the rising public demand for high- quality, inexpensive healthcare services, is driving the growth of healthcare products, therefore, hospital pharmacy would generate more than 55% share of the market by 2030. The increasing number & size of investments invested in the healthcare industry by governments of different nations is one of the primary drivers of the market’s growth. The WHO reports that healthcare spending reached a record high of US $9 trillion (about 11% of global GDP) due to substantial increases in healthcare spending by governments at all income levels. In general, rising government health spending largely offsets declining personal spending. Increasing spending in the healthcare industry has led to an increase in market innovations. Some of the factors impacting the market are increase in elderly population, growing awareness of AMD, and Wet AMD drugs' adverse effects.
As it gets worse with time, vision loss in the elderly is a major issue. The two main types of macula deterioration are wet and dry macular degeneration. Both are possibly fatal and require medical care. According to the WHO, there will be 1.4 billion people aged 60 and older by 2050, up from the current 1 billion. The number of individuals 60 and older worldwide will double (to 2.1 billion) by 2050. Some key players are working on vitreous implants and longer-acting anti-VEGFs that enable sustained medication delivery. Additionally, the most frequent cause of permanent vision loss is macular degeneration. To make the public aware of the effects of AMD and the best treatment options, NGOs, optometrist groups, and health agencies run various programs, activities, and campaigns. The National AMD Awareness Month celebrated in February and strives to increase awareness of AMD as the main cause of visual loss, is one such significant initiative. Rising financing for the condition's research and related activities, which also encourage people to have eye exams to improve the likelihood of early identification, is another factor driving the need for macular degeneration drugs.
However, wet AMD medicines' wide range of negative effects constrains the market's growth. Growth is expected to be restricted by side effects such as retinal vasculitis, intraocular inflammation, concomitant vascular occlusion, nausea, vomiting, stopping loss of vision in 90% of cases, bleeding in the eye, redness & irritation of the eye, and individuals losing their vision permanently. High blood pressure and inflammation of the nose and throat are also prevalent. Furthermore, these medications are injected directly into the eye, which carries potential hazards. These issues may hamper market expansion. Moreover, as the COVID-19 pandemic had significant effects on healthcare systems globally, including the treatment and management of chronic diseases like age-related macular degeneration (AMD), it had a negative impact on the age-related degeneration (AMD) market. Due to clinic closures and reduced hospital capacity during the outbreak, many people could not get basic eye care and treatment. However, the pandemic impacted the development and approval of new treatments for age-related macular degeneration (AMD), which has had a negative effect on the expanding AMD market.
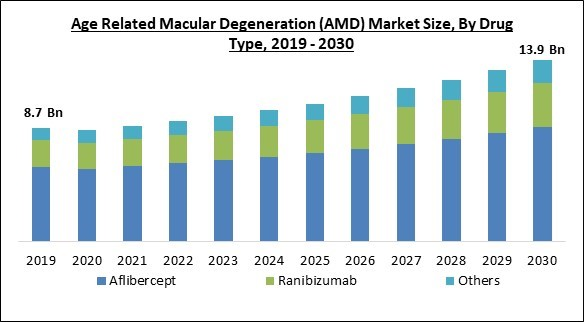
Drug Type Outlook
On the basis of drug type, the market is segmented into aflibercept, ranibizumab, and others. In 2022, the aflibercept segment dominated the market with the maximum revenue share. This segment will expand due to the increased use of the medicine Aflibercept globally and the rise in age-related macular degeneration (AMD) among the older population.Disease Type Outlook
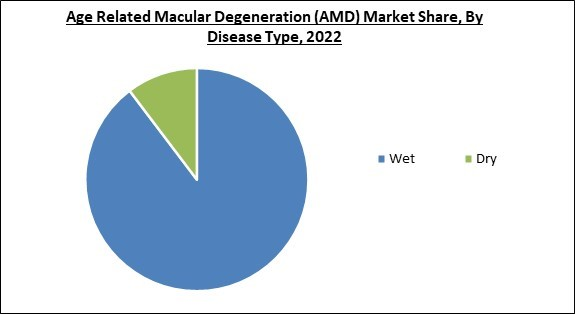
Based on diseases type, the market is classified into wet, and dry. The dry segment garnered a significant revenue share in the market in 2022. Dry AMD causes 85 to 90% of all cases of macular degeneration. Vitamin formulations are the only treatment options for dry macular degeneration. Luminate, Zimura, and intravitreal pegcetacoplan are only a few of the cutting-edge therapeutic approaches for its treatment that are now being tested in clinical settings. Accepting these candidates could accelerate the dry AMD segment's expansion in the upcoming years.Distribution Channel Outlook
By distribution channel, the market is categorized into hospital pharmacy, retail pharmacy, and online pharmacy. The online segment recorded a remarkable revenue share in the market in 2022. The segment's growth is primarily driven by rising internet usage, the digitization of healthcare services, and an increase in tech-savvy consumers worldwide. Consumers' desire for online shopping, which is increasingly centered on convenience, is also a factor in the increase.Regional Outlook
Region wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The Asia Pacific region covered a considerable revenue share in the market in 2022. The sale of medications for exudative AMD is now very low in APAC because of problems with accessibility and expensive prescriptions. However, AMD is highly prevalent in this region, making it a potentially growing market. A few companies in APAC are also involved in the marketing and research of exudative AMD therapies. In addition, the demand for wet age-related macular degeneration drugs is expected to rise considerably in the coming years due to the region accounting for more than a third of all age-related macular degeneration cases globally.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Biogen, Inc., Bayer AG, Apellis Pharmaceuticals, Inc., Regeneron Pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., Ionis Pharmaceuticals, Inc., Novartis AG, Sanofi S.A., Coherus Biosciences, Inc. and Bausch Health Companies, Inc.
Strategies Deployed in the Market
- Jun-2023: Bausch + Lomb introduced PreserVision AREDS 2 Formula soft gels plus coenzyme Q10 (CoQ10) in the United States. It is the only eye vitamin that combines the precise nutrient formula advised by the National Eye Institute (NEI) to help reduce the risk of moderate to advanced Age-related Macular Degeneration (AMD) progression in AMD patients with CoQ10 to support heart health.
- Feb-2023: Apellis Pharmaceuticals received approval from the U.S. Food & Drug Administration (FDA) for SYFOVRE™ (pegcetacoplan injection). This injection is launched for people with geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
- Aug-2022: Coherus BioSciences announced that the U.S. Food and Drug Administration (FDA) approved CIMERLITM (ranibizumab-eqrn) as a biosimilar product interchangeable with Lucentis® (ranibizumab injection) for all five indications. This product satisfies all of the FDA's strict requirements for the reference product, including those for quality, safety, and efficacy. The anti-VEGF therapeutic class of biologics, which includes CIMERLITM, has revolutionized how retinal patients can keep or regain eyesight.
- Jun-2022: Biogen together with Samsung Bioepis Co., Ltd. launched BYOOVIZ™ (ranibizumab-nuna), a biosimilar referencing LUCENTIS® (ranibizumab)i in the United States. The biosimilar has been approved by FDA and it would be utilized for the treatment of macular edema following retinal vein occlusion, neovascular (wet) age-related macular degeneration (AMD), and myopic choroidal neovascularization. This is an affordable treatment option for patients suffering from retinal vascular disorders.
- Feb-2022: Novartis completed the acquisition of Gyroscope Therapeutics, a clinical-stage gene therapy company. With this acquisition, Novartis would take another step toward its goal of providing ophthalmic innovation to treat and prevent blindness globally. The acquisition strengthened its current expertise in retinal disorders and gene therapy.
- Oct-2020: Novartis took over Vedere Bio, a growing pharmaceutical company. The acquisition strengthened Novartis' dedication to cell and gene therapy and helped Novartis accelerate its efforts to provide revolutionary treatments to a variety of patients with blinding conditions. Additionally, the acquisition increased Novartis' presence in ophthalmology and strengthened its position as a global leader in AAV-based gene therapy.
- Oct-2019: Novartis got the U.S. Food and Drug Administration approval for Beovu® (brolucizumab) injection, also known as RTH258. This injection would be utilized in the treatment of wet age-related macular degeneration (AMD). Beovu is the only anti-VEGF licensed by the FDA to provide both better fluid resolution compared to aflibercept and the capacity to keep eligible wet AMD patients on a three-month dosage interval immediately following a three-month loading phase with uncompromised efficacy.
Scope of the Study
Market Segments Covered in the Report:
By Drug Type
- Aflibercept
- Ranibizumab
- Others
By Disease Type
- Wet
- Dry
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Biogen, Inc.
- Bayer AG
- Apellis Pharmaceuticals, Inc.
- Regeneron Pharmaceuticals, Inc.
- F.Hoffmann-La Roche Ltd.
- Ionis Pharmaceuticals, Inc.
- Novartis AG
- Sanofi S.A.
- Coherus Biosciences, Inc.
- Bausch Health Companies, Inc.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Biogen, Inc.
- Bayer AG
- Apellis Pharmaceuticals, Inc.
- Regeneron Pharmaceuticals, Inc.
- F. Hoffmann-La Roche Ltd.
- Ionis Pharmaceuticals, Inc.
- Novartis AG
- Sanofi S.A.
- Coherus Biosciences, Inc.
- Bausch Health Companies, Inc.