Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Increasing Prevalence of Cardiovascular Diseases
The increasing prevalence of cardiovascular diseases is a major growth driver in the Canadian cardiovascular devices market. Cardiovascular diseases are a leading cause of death in Canada, and the aging population and unhealthy lifestyle habits, such as poor diet and lack of exercise, are further contributing to the rising prevalence of these diseases. As the number of patients with cardiovascular diseases increase, the demand for cardiovascular devices is also increasing. This includes devices such as implantable cardioverter defibrillators (ICDs), pacemakers, cardiac resynchronization therapy (CRT) devices, and transcatheter heart valves.The Canadian healthcare system is also investing heavily in the efficient diagnosis and treatment of cardiovascular diseases. This is driving the adoption of advanced technologies and treatments, including the use of remote patient monitoring and minimally invasive procedures. Furthermore, the Canadian government is promoting the growth of the cardiovascular devices market by supporting research and development in this field. For instance, the Canadian Institutes of Health Research (CIHR) has invested in several initiatives aimed at improving cardiovascular health, including the development of new devices and treatments.
Technological Advancements
Technological advancements have a significant impact on the growth of the Canadian cardiovascular devices market. Advances in minimally invasive procedures have reduced the need for open-heart surgery, resulting in shorter hospital stays, faster recovery times, and lower healthcare costs. These procedures rely on advanced imaging techniques, such as 3D imaging, to guide the placement of cardiovascular devices such as stents, pacemakers, and heart valves. Remote patient monitoring technologies, such as wireless implantable sensors, enable healthcare professionals to remotely monitor patient health, and detect potential cardiovascular events before they become serious. This technology allows patients to receive more personalized and proactive care, improving patient outcomes, and reducing healthcare costs. AI is being increasingly used in the diagnosis and treatment of cardiovascular diseases. Machine learning algorithms can analyze large amounts of patient data to identify patterns and predict the likelihood of cardiovascular events.Wearable devices, such as smartwatches and fitness trackers, are becoming increasingly popular for monitoring heart rate, blood pressure, and other cardiovascular health metrics. These devices are affordable, easy to use, and can provide patients with real-time feedback on their health, enabling them to take a more active role in managing their cardiovascular health. 3D printing technology is being used to create custom-made cardiovascular devices, such as heart valves and stents, that fit a patient's unique anatomy. This technology can reduce the risk of complications and improve patient outcomes.
Growing Adoption of Remote Patient Monitoring
The growing adoption of Remote Patient Monitoring (RPM) is having a significant impact on the growth of the Canadian cardiovascular devices market. RPM involves monitoring patient health remotely, enabling healthcare professionals to provide more personalized care, and improve patient outcomes. In the context of cardiovascular health, RPM can involve the use of wearable devices, implantable sensors, and other technologies to monitor metrics such as heart rate, blood pressure, and cholesterol levels. This data can be transmitted to healthcare professionals in real-time, allowing for early detection of potential cardiovascular events and more proactive treatment. The adoption of RPM in Canada is being driven by several factors, including the aging population, rising prevalence of chronic diseases, and advancements in technology. Patients with cardiovascular diseases are often required to monitor their health closely, which can be challenging and time-consuming. RPM technologies provide patients with a more convenient and streamlined way to manage their health, improving patient engagement and adherence to treatment plans. RPM also has the potential to reduce healthcare costs by enabling earlier detection of cardiovascular events and reducing the need for hospital readmissions. This is particularly important in Canada's publicly funded healthcare system, which is currently under increasing pressure to reduce costs while maintaining high-quality care. The adoption of RPM is creating new growth opportunities for companies operating in the Canadian cardiovascular devices market. For instance, manufacturers of implantable sensors and wearable devices such as Abbott and GE healthcare, are experiencing increased demand for their products. Additionally, companies that offer RPM software and analytics solutions are benefiting from the growing demand for data-driven healthcare solutions.Government Support
Government support plays a crucial role in the growth of the Canadian cardiovascular devices market. The Canadian government has implemented several initiatives to support the development and adoption of cardiovascular devices, creating a favorable environment for companies operating in this field. One key initiative is the Scientific Research and Experimental Development (SR&ED) tax credit program. This program provides tax incentives to companies that invest in research and development activities in Canada, including the development of new cardiovascular devices. This support has helped to attract investment in the Canadian cardiovascular devices market, encouraging companies to pursue new product development and innovation. The Canadian government has also made significant investments in healthcare infrastructure, including the development of cardiovascular centers of excellence across the country. These centers provide specialized care for patients with cardiovascular diseases and serve as hubs for the development and adoption of new cardiovascular devices and procedures. The government also plays a role in regulating the cardiovascular devices market. Health Canada is responsible for ensuring the safety and efficacy of medical devices in Canada, including cardiovascular devices. The regulatory process is designed to ensure that new devices are safe and effective before they are allowed on the market, providing assurance to patients and healthcare professionals. In addition to these initiatives, the Canadian government provides funding for healthcare research and innovation through organizations such as the Canadian Institutes of Health Research (CIHR) and the National Research Council of Canada (NRC). This funding helps to support the development of new cardiovascular devices and procedures, as well as research into the causes and treatment of cardiovascular diseases.Increasing Awareness
Increasing awareness of cardiovascular diseases and the importance of early detection and treatment is having a significant impact on the growth of the Canadian cardiovascular devices market. As more people become aware of the risks associated with cardiovascular diseases, they are seeking out preventive measures and treatment options, driving the demand for cardiovascular devices. Awareness of cardiovascular diseases is also being driven by factors like public health campaigns and media coverage. The Canadian government and non-profit organizations have launched public health campaigns to raise awareness about the risk factors associated with cardiovascular diseases, such as high blood pressure, smoking, and poor diet. Increased awareness has also led to more screening programs and early detection of cardiovascular diseases. This has created a growing market for diagnostic devices such as blood pressure monitors, electrocardiographs, and imaging equipment used to identify and diagnose cardiovascular diseases. Additionally, awareness has led to increased demand for innovative treatment options, such as minimally invasive procedures and implantable devices. These devices include pacemakers, implantable defibrillators, and heart valves, among others. The growing awareness of cardiovascular diseases is creating new growth opportunities for companies operating in the Canadian cardiovascular devices market. Companies that offer innovative and effective treatment options are experiencing increased demand for their products. Additionally, companies that specialize in diagnostic devices and remote monitoring technologies are benefiting from the growing demand for early detection and proactive management of cardiovascular diseases.Recent Development
- Medtronic’s Micra AV: In 2021, Medtronic launched the Micra AV, a miniaturized pacemaker designed to provide atrioventricular (AV) synchrony without the need for a lead in the heart. The device is less than one-tenth the size of a traditional pacemaker and can be implanted directly into the heart using a minimally invasive procedure.
- Abbott’s MitraClip G4: In 2020, Abbott launched the MitraClip G4, a minimally invasive device used to repair a leaky mitral valve in the heart. The device can be implanted using a catheter-based procedure, avoiding the need for open-heart surgery.
- Boston Scientific’s Watchman FLX: In 2019, Boston Scientific launched the Watchman FLX, a device used to reduce the risk of stroke in patients with atrial fibrillation. The device is implanted in the heart using a minimally invasive procedure and blocks the left atrial appendage, where blood clots can form and lead to stroke.
Market Segmentation
The Canada Cardiovascular Devices Market can be segmented by Device Type, Application, End User and Region. Based on Device Type, the market can be divided into Diagnostic and Monitoring Devices v/s Surgical Devices. Based on Application, the market can be segmented into Coronary Artery Disease, Cardiac Arrhythmia, Heart Failure, and Others. Based on End User, the market can be grouped into Hospitals, Diagnostic Centers, and Others.Market Players
Abbott Canada, Cardinal Health Canada Inc., GE Healthcare Canada Inc, W L Gore & Associates Canada Inc, Medtronic of Canada Ltd, Biotronik Canada Inc, Siemens Healthcare Limited., Canon Medical Systems Canada Ltd., B. Braun of Canada., and LivaNova PLC. are some of the leading players operating in the Canada cardiovascular devices market.Report Scope
In this report, the Canada Cardiovascular Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Canada Cardiovascular Devices Market, by Device Type:
- Diagnostic and Monitoring Devices
- Surgical Devices
Canada Cardiovascular Devices Market, by Application:
- Coronary Artery Disease
- Cardiac Arrhythmia
- Heart Failure
- Others
Canada Cardiovascular Devices Market, by End User:
- Hospitals
- Diagnostic Centers
- Others
Canada Cardiovascular Devices Market, by Region:
- Ontario region
- Quebec region
- Alberta region
- British Columbia region
- Saskatchewan and Manitoba region
- Rest of Canada
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in Canada Cardiovascular Devices Market.Available Customizations
The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott Canada
- Cardinal Health Canada Inc.
- GE Healthcare Canada Inc.
- W L Gore & Associates Canada Inc.
- Medtronic of Canada Ltd.
- Biotronik Canada Inc.
- Siemens Healthcare Limited.
- Canon Medical Systems Canada Ltd.
- B. Braun of Canada.
- LivaNova PLC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 79 |
| Published | August 2023 |
| Forecast Period | 2022 - 2028 |
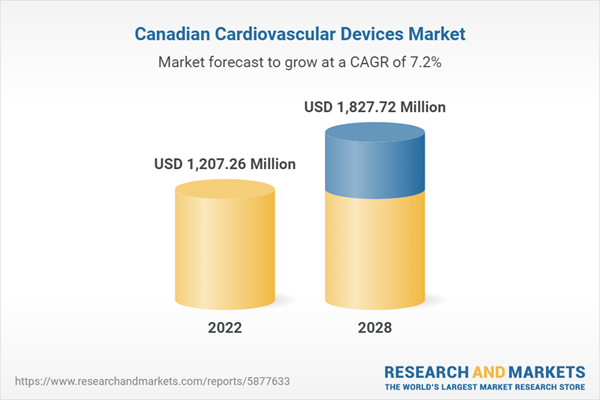
| Estimated Market Value ( USD | $ 1207.26 Million |
| Forecasted Market Value ( USD | $ 1827.72 Million |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Canada |
| No. of Companies Mentioned | 10 |