Amniotic Membrane Global Market Report by Product (Cryopreserved amniotic membranes, Lyophilization Amniotic Membrane), Application (Surgical Wounds, Ophthalmology, Others), End User (Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Research & Academic Institutes), Countries and Company Analysis, 2025-2033.
Global Amniotic Membrane Industry Overview
The thick foundation membrane and avascular stromal matrix make up the amniotic membrane, the placenta's deepest layer. Because of its special qualities, this biological tissue - which comes from the placenta - is used in medicine. It lessens tissue fibrosis and scarring and lowers inflammation by regulating inflammatory responses. It speeds up the healing process and has a calming effect on burns and severe wounds. It is frequently utilized in procedures for conditions of the ocular surface, including corneal ulcers, and is well-tolerated and safe with low rejection rates. In order to improve healing and lessen adhesions, it is also used as a biological dressing.Amniotic membranes are commonly employed in two ways to heal and manage wounds: as a biological bandage or as a surgical graft. When utilized as a graft, these membranes operate as a scaffold to promote re-epithelization. Its anti-inflammatory and anti-scarring qualities are particularly important when burn injuries occur since they help patients feel less uncomfortable and less inflamed. Therefore, it is expected that the growing advantages of amniotic membranes will drive market expansion. Amniotic membrane therapy is frequently used to address conditions affecting the surface of the eye. treatment for a number of conditions, including ocular dystrophy, cataracts, glaucoma, bullous keratopathy, corneal degeneration, bacterial keratitis, corneal ulcers, eyelid reconstruction, and more. There is an enormous demand for these tissue-based products because to the increase in ophthalmology surgery brought on by the global aging population.
Verséa unveiled a point-of-care platform for amniotic membrane transplantation in February 2024. By offering instant diagnostic results and treatment alternatives, this innovation improves patient care in ophthalmology by facilitating quick and efficient testing.
Woo University and NovaBay Pharmaceuticals collaborated in December 2023 to train eyecare providers on the application of amniotic membranes. The collaboration includes a free webinar that offers continuing education credits and covers clinical uses, patient selection, and billing for amniotic membranes, specifically Avenova Allograft from NovaBay, which helps restore the ocular surface.
Growth Drivers for the Amniotic Membrane Market
Technological developments in healthcare
Amniotic membranes are becoming more effective and efficient as a result of the incorporation of cutting-edge technologies. Their application in a variety of medical sectors is increased by modern approaches that guarantee the preservation of vital growth factors and therapeutic capabilities. A promising commercial outlook is being provided by the amniotic membrane's compatibility with innovative therapies like stem cell therapies and three-dimensional (3D) bioprinting. These linkages improve the standard of patient care by enabling more individualized and efficient treatments. In addition, better preservation techniques provide a longer shelf life and more dissemination, which is driving up its uptake in different geographical areas and healthcare systems.Favorable regulatory framework
Many nations' regulatory agencies are establishing precise and well-defined protocols for the extraction, processing, preservation, and use of amniotic membranes as a result of their recognition of the membrane's medicinal usefulness. These rules maintain product quality and guarantee consistency in procedures. In addition, regulatory bodies keep a close eye on the market to make sure that all applicable laws and guidelines are being followed. The industry's integrity and confidence are preserved by routine audits, inspections, and reporting obligations. Additionally, they are providing supportive policies that promote research and development in the field, such as grants, incentives, and public-private partnerships. Working together with research organizations can spur innovation and introduce cutting-edge goods and treatments to the market.Growing interest in regenerative medicine
The demand for efficient treatment strategies is growing as a result of the increased incidence of many chronic diseases, such as diabetes and rheumatoid arthritis, which can cause ulcers and chronic wounds. Amniotic membranes promote the healing process and provide a novel solution. In addition, the need for therapies that encourage quicker healing and tissue regeneration is being driven by the world's aging population, which is becoming more and more vulnerable to a number of illnesses. Additionally, the market is being positively impacted by the growing use of amniotic membranes in a variety of medical disciplines, such as dentistry and orthopedics. Furthermore, the amniotic membrane's capacity to promote cellular proliferation and reduce inflammation is propelling its use in other regenerative therapies.Challenges in the Amniotic Membrane Market
Regulatory Hurdles
Amniotic membrane products are difficult to regulate because of the intricate rules established by organizations such as the FDA. The approval and marketing of amniotic membranes are complicated by the fact that they are subject to both medical device regulations and human tissue laws. Strict regulations for donor screening, tissue processing, labeling, and tracking must be followed by businesses; these regulations can differ depending on the location. These regulatory variations may raise operating expenses and postpone market entry. The strain is further increased by the requirement for strict documentation and quality control. Because of this, companies have a difficult time maintaining compliance while attempting to satisfy consumer needs. Companies hoping to commercially market amniotic membrane-based products worldwide must overcome these regulatory obstacles.Storage and Shelf-Life Concerns
Due to their limited shelf life as biological materials, amniotic membranes provide storage and transportation issues. For these products to remain viable and functional, certain conditions like freezing or cryopreservation are frequently needed. The supply chain becomes much more expensive and logistically difficult as a result. Furthermore, the broad availability of amniotic membrane products is restricted by the requirement for specialized storage infrastructure, such as temperature-controlled facilities, especially in areas with low resources. These elements raise operating expenses and can make it challenging to continuously supply demand, particularly in rural or developing regions. Improving access and reaching a wider audience need addressing these shelf-life and storage issues.United States Amniotic Membrane Market
Because of its uses in tissue regeneration, ophthalmology, and wound healing, the amniotic membrane market in the US has grown significantly. The market gains from the rising demand for innovative therapies and regenerative medicine. The increasing frequency of chronic diseases including diabetes and eye disorders, as well as the growing need for minimally invasive therapies, are major factors propelling this expansion. Obstacles include high production costs, regulatory constraints, and moral dilemmas around the use of tissues originating from humans. Notwithstanding these obstacles, the industry is still growing as a result of new technology and rising provider awareness. To get beyond these obstacles and take advantage of the prospects in regenerative medicine, American businesses are spending money on research, product development, and strategic alliances.In March 2023, Amaro Law Firm released research stating that 7.3 million motor vehicle accidents occur in the United States each year. Therefore, it is projected that over the forecast period, the rising incidence of accidents will increase demand for amniotic membrane.
United Kingdom Amniotic Membrane Market
The market for amniotic membranes in the UK is expanding due to developments in regenerative medicine, namely in the areas of tissue repair, ophthalmology, and wound healing. Demand is rising as a result of a preference for biologic treatments and an increase in the prevalence of chronic disorders including diabetic ulcers and eye problems. Regulatory complexity, high production costs, and restricted donor tissue availability are some of the market's obstacles, though. Market expansion is also impacted by ethical difficulties around the use of tissues originating from humans. Notwithstanding these challenges, the UK market is bolstered by a robust healthcare system, growing knowledge of the uses of amniotic membranes, and continuous studies into their effectiveness. With an emphasis on increasing product accessibility and reducing prices for wider clinical use, there are prospects for additional growth as the use of regenerative therapies increases.More than 500,000 people in the UK already have venous ulcers of some kind, a number that has doubled over the last ten years, according to data released by The Primary Care Dermatology Society in October 2023. The need for sophisticated wound care treatments, such as amniotic membrane products, has grown dramatically as a result of the increase in instances. These membranes' ability to accelerate healing and lower infection rates makes them an essential part of treating venous leg ulcers, which propels market expansion and provider adoption.
India Amniotic Membrane Market
The growing need for regenerative treatments in ophthalmology, wound healing, and tissue regeneration is driving the amniotic membrane market's expansion in India. This need is being driven by rising rates of chronic diseases like diabetes, diabetic ulcers, and eye ailments. Regulatory complexity, high production costs, and restricted donor tissue availability are some of the market's obstacles, though. Furthermore, acceptance in some areas may be impacted by ethical issues around the usage of items originating from humans. Notwithstanding these obstacles, India's developing healthcare system and rising awareness of cutting-edge treatments are driving the industry. Significant prospects are presented by the growing use of regenerative medicine, and future market growth is anticipated to be driven by continued research into more affordable treatments and easier access to products.According to Hindustan Times in August 2023, the market for amniotic membranes in India is expanding significantly as a result of a 70% increase in eye infections. Viral and bacterial illnesses have increased as a result of poor sanitary conditions brought on by recent rains and floods. Experts in medicine point to the growing need for amniotic membranes, which have anti-inflammatory and regenerative qualities, in the treatment of conditions affecting the ocular surface.
Saudi Arabia Amniotic Membrane Market
The rising need for cutting-edge regenerative therapies in tissue regeneration, ophthalmology, and wound healing is propelling the amniotic membrane market in Saudi Arabia. The market is expanding due to factors like the increased incidence of chronic illnesses like diabetes and eye disorders. But there are still obstacles like complicated regulations, expensive production, and a shortage of donor tissues. Acceptance may also be influenced by ethical considerations related to the usage of items derived from humans. Notwithstanding these obstacles, the market is expected to develop due to Saudi Arabia's established healthcare system, rising healthcare spending, and rising awareness of regenerative medicine. Further investigation and cooperation with global partners may aid in removing obstacles, promoting growth and opening up amniotic membrane products to a wider range of patients.This growth is anticipated to be driven by the increase in healthcare spending, which is predicted to reach USD 50.4 billion in 2023, according to a report by the U.S. Department of Commerce and the International Trade Administration. Another important element driving market expansion is the growing number of hospitals and medical professionals.
Amniotic Membrane Market Segments
Product-Market breakup in 2 viewpoints:
1. Cryopreserved amniotic membranes2. Lyophilization Amniotic Membrane
Application- Market breakup in 3 viewpoints:
1. Surgical Wounds2. Ophthalmology
3. Others
End User - Market breakup in 4 viewpoints:
1. Hospitals2. Ambulatory Surgical Centers
3. Specialty Clinics
4. Research & Academic Institutes
Country
1. United States2. Canada
3. Germany
4. United Kingdom
5. France
6. Italy
7. Netherlands
8. Spain
9. China
10. South Korea
11. Japan
12. India
13. Indonesia
14. Malaysia
15. Argentina
16. Brazil
17. Mexico
18. Colombia
19. Saudi Arabia
20. South Africa
21. Israel
22. Australia
23. UAE
24. ROW
All the Key players have been covered from 4 Viewpoints:
1. Overview2. Key Persons
3. Recent Developments
4. Financial Insights
Company Analysis:
1. Stryker Corporation2. Smith & Nephew plc
3. Integra LifeSciences Holdings
4. MiMedx
5. Organogenesis Net
6. Wright Medical Group Inc
7. Applied Biologics LLC
8. FzioMed Inc
9. Katena, Inc.
10. Tissue-Tech Inc
Table of Contents
Companies Mentioned
- Stryker Corporation
- Smith & Nephew plc
- Integra LifeSciences Holdings
- MiMedx
- Organogenesis Net
- Wright Medical Group Inc
- Applied Biologics LLC
- FzioMed Inc
- Katena, Inc.
- Tissue-Tech Inc
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2024 - 2033 |
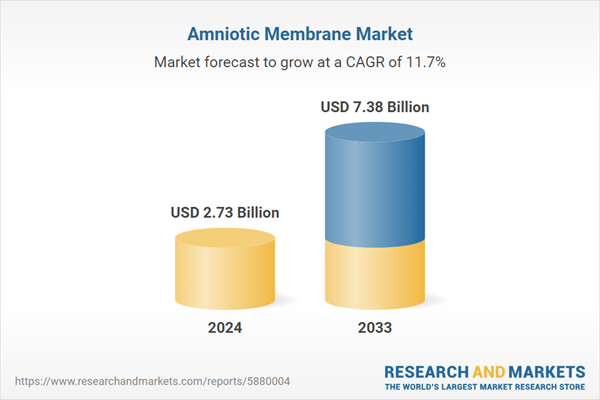
| Estimated Market Value ( USD | $ 2.73 Billion |
| Forecasted Market Value ( USD | $ 7.38 Billion |
| Compound Annual Growth Rate | 11.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |