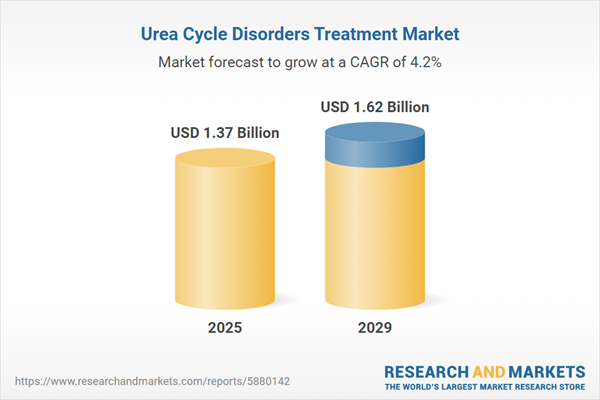
The urea cycle disorders treatment market size is expected to see steady growth in the next few years. It will grow to $1.62 billion in 2029 at a compound annual growth rate (CAGR) of 4.2%. The growth in the forecast period can be attributed to advancements in enzyme replacement therapy, expanded newborn screening programs, increased collaboration in research, expansion of therapeutic options, telemedicine and remote care. Major trends in the forecast period include advanced gene editing techniques, awareness and advocacy initiatives, regenerative medicine approaches, long-term safety and efficacy studies, and personalized nutritional therapies.
The forecast of 4.2% growth over the next five years reflects a modest reduction of 0.2% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Trade tensions may burden U.S. metabolic genetics clinics by inflating costs of nitrogen scavenging drugs (sodium phenylbutyrate/glycerol phenylbutyrate) sourced from Israel and Canada, potentially reducing urea cycle disorder management options and elevating inborn error of metabolism treatment expenses. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The increasing prevalence of urea cycle disorders is projected to drive the growth of the urea cycle disorders treatment market in the future. Urea cycle disorders refer to metabolic conditions that are inherited and result from defects in one of the enzymes or transporter molecules essential for the liver's removal of ammonia from the bloodstream. The rise in these disorders is primarily attributed to genetic factors. When ammonia accumulation is identified as a urea cycle disorder (UCD), a tailored treatment approach involving dietary management, medication, and monitoring can be established to mitigate its adverse effects. For example, in April 2023, data from StatPearls Publishing LLC, a US-based medical education resource, indicated that the prevalence of urea cycle disorders due to ornithine transcarbamylase deficiency is 1 in 140,000 individuals. Additionally, in September 2022, according to Lecturio, a Germany-based digital medical education platform, Urea cycle disorders (UCDs) occur in about 1 in every 35,000 live births globally and 1 in 8,200 live births in the United States, resulting in an annual incidence of roughly 113 new cases in the U.S. and around 149 in Europe. Therefore, the rising prevalence of urea cycle disorders is fueling the growth of the urea cycle disorders treatment market.
The increasing number of chronic kidney disorder cases is expected to contribute to the expansion of the urea cycle disorders treatment market. Chronic kidney disease (CKD), characterized by the gradual loss of kidney function over time, utilizes urea cycle disorder treatment to manage metabolic waste, especially elevated urea levels due to impaired kidney function. As of November 2023, an estimated 7.2 million individuals are believed to have chronic kidney disease (CKD) stages 1-5, with approximately 3.5 million people in the UK experiencing the later stages of CKD (stages 3-5), where symptoms become progressively challenging to control. Hence, the growing number of chronic kidney disorder cases is a driving force behind the urea cycle disorders treatment market's growth.
A product innovation has emerged as a notable trend, with major companies focusing on creating innovative products to strengthen their market positions. For example, in September 2022, Medunik USA introduced Pheburane oral pellets, a unique and flavor-masking formulation of sodium phenylbutyrate (NaPB) for the long-term management of certain urea cycle disorders. Pheburane, a prescription drug used in conjunction with a specific diet, offers a convenient administration method without the need for mixing. It can be consumed alone or sprinkled on food, enhancing patient compliance.
Major companies in the urea cycle disorder treatment sector are embracing a strategic partnership approach to enhance innovative therapies and improve disease management. Strategic partnerships involve companies utilizing each other's strengths and resources to achieve mutual advantages and success. For example, in June 2024, Orsini Specialty Pharmacy, a US-based provider of personalized care and medications, and Zevra Therapeutics Inc., a US-based company focused on rare disease therapeutics, announced that Orsini will serve as the pharmacy partner for OLPRUVA (sodium phenylbutyrate) oral suspension. OLPRUVA is a prescription medication used alongside specific therapies, including dietary modifications, for the long-term management of urea cycle disorders (UCDs) in particular adult and pediatric patients with deficiencies in carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
In November 2023, Zevra Therapeutics Inc. acquired Acer Therapeutics Inc. for $91 million, providing Zevra access to rare disease assets, including Edsivo and Olpruva. Edsivo is undergoing Phase III trials for vascular Ehlers-Danlos syndrome, while Olpruva has received FDA approval for certain urea cycle diseases. This acquisition enhances Zevra's market presence and diversifies its revenue stream in the rare disease therapeutics sector. Acer Therapeutics Inc. is a pharmaceutical company offering treatments for urea cycle disorders.
Major companies operating in the urea cycle disorders treatment market include Bausch Health Companies Inc., Recordati Rare Diseases, Eurocept Pharmaceuticals Holding (Lucane Pharma SA), Acer Therapeutics, Ultragenyx Pharmaceutical, Aeglea BioTherapeutics, Arcturus Therapeutics, Inc., Orpharma Pty Ltd., Selecta Biosciences, Inc., Abbott Laboratories, Mead Johnson & Company, LLC, Horizon Therapeutics Plc, Nestlé S.A., Danone S.A., Biophone, Relief Therapeutics Holding AG, TheraTriage, Genomatica, Protalix BioTherapeutics, Erytech Pharma, 4D Pharma, Synlogic, Amicus Therapeutics, Sobi, Astellas Pharma Inc., Chiesi Farmaceutici S.p.A., Medpace Holdings, Inc., Celerion, and PTC Therapeutics.
North America was the largest region in the urea cycle disorders treatment market in 2024. The regions covered in the urea cycle disorders treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the urea cycle disorders treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The urea cycle disorders treatment market research report is one of a series of new reports that provides urea cycle disorders treatment market statistics, including urea cycle disorders treatment industry global market size, regional shares, competitors with a urea cycle disorders treatment market share, detailed urea cycle disorders treatment market segments, market trends and opportunities, and any further data you may need to thrive in the urea cycle disorders treatment industry. This urea cycle disorders treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Urea cycle disorder treatment involves managing rare genetic diseases in which the body lacks the necessary enzymes to break down ammonia, leading to its accumulation and potential toxicity. The primary goal of urea cycle disorder treatment is to reduce blood ammonia levels.
The main types of treatment for urea cycle disorders include amino acid supplements, sodium phenylbutyrate, glycerol phenylbutyrate, sodium benzoate, and others. Amino acid supplements contain one or more of the nine essential amino acids that the body requires but cannot produce independently. Specific types of enzyme deficiencies are addressed, such as OTC (ornithine transcarbamylase), AS (argininosuccinate synthetase) for citrullinemia, AG (arginase), AL (argininosuccinate lyase), and CPS1 (carbamoyl phosphate synthase). These treatments can be administered orally and through injections, and they are distributed through various channels, including hospital pharmacies, retail pharmacies, and online pharmacies.
The urea cycle disorders treatment market includes revenues earned by entities by hemodialysis, dietary management, and genetic counselling. The market value includes the value of related goods sold by the service provider or included within the service offering. The urea cycle disorders treatment market also includes sales of arginine, sodium phenylacetate, sodium benzoate, and carglumic acid. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Urea Cycle Disorders Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on urea cycle disorders treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for urea cycle disorders treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The urea cycle disorders treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Treatment: Amino Acid Supplements; Sodium Phenylbutyrate; Glycerol Phenylbutyrate; Sodium Benzoate; Other Treatments2) By Enzyme Deficiency: Ornithine Transcarbamylase (OTC); Argininosuccinate Synthetase (AS); Arginase (AG); Argininosuccinate Lyase (AL); Carbamoyl Phosphate Synthase (CPS1)
3) By Route of Administration: Oral; Injectables
4) By Distribution Channel: Hospital Pharmacies; Retail Pharmacies; Online Pharmacies
Subsegments:
1) By Amino Acid Supplements: Arginine Supplements; Citrulline Supplements; Other Essential Amino Acids2) By Sodium Phenylbutyrate: Powder Form; Tablet Form
3) By Glycerol Phenylbutyrate: Oral Solutions; Other Forms
4) By Sodium Benzoate: Intravenous Solutions; Oral Formulations
5) By Other Treatments: Liver Transplantation; Dietary Modifications; Enzyme Replacement Therapy; Other Supportive Therapies
Companies Mentioned: Bausch Health Companies Inc.; Recordati Rare Diseases; Eurocept Pharmaceuticals Holding (Lucane Pharma SA); Acer Therapeutics; Ultragenyx Pharmaceutical; Aeglea BioTherapeutics; Arcturus Therapeutics, Inc.; Orpharma Pty Ltd.; Selecta Biosciences, Inc.; Abbott Laboratories; Mead Johnson & Company, LLC; Horizon Therapeutics Plc; Nestlé S.A.; Danone S.A.; Biophone; Relief Therapeutics Holding AG; TheraTriage; Genomatica; Protalix BioTherapeutics; Erytech Pharma; 4D Pharma; Synlogic; Amicus Therapeutics; Sobi; Astellas Pharma Inc.; Chiesi Farmaceutici S.p.A.; Medpace Holdings, Inc.; Celerion; and PTC Therapeutics
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Urea Cycle Disorders Treatment market report include:- Bausch Health Companies Inc.
- Recordati Rare Diseases
- Eurocept Pharmaceuticals Holding (Lucane Pharma SA)
- Acer Therapeutics
- Ultragenyx Pharmaceutical
- Aeglea BioTherapeutics
- Arcturus Therapeutics, Inc.
- Orpharma Pty Ltd.
- Selecta Biosciences, Inc.
- Abbott Laboratories
- Mead Johnson & Company, LLC
- Horizon Therapeutics Plc
- Nestlé S.A.
- Danone S.A.
- Biophone
- Relief Therapeutics Holding AG
- TheraTriage
- Genomatica
- Protalix BioTherapeutics
- Erytech Pharma
- 4D Pharma
- Synlogic
- Amicus Therapeutics
- Sobi
- Astellas Pharma Inc.
- Chiesi Farmaceutici S.p.A.
- Medpace Holdings, Inc.
- Celerion
- and PTC Therapeutics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.37 Billion |
| Forecasted Market Value ( USD | $ 1.62 Billion |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |