Use of Modern Technologies in CIN & HR-HPV Screening and Diagnosis is Fuelling Asia Pacific CIN & HR-HPV Treatment Market
Health authorities across the region recommend routine cervical cancer screenings to women as ~30% of the grade 3 CIN lesions develop into invasive cancers within 30 years. Slow progression provides several opportunities for detection and treatment. The manual cervical cancer screening methods are not always accurate, which results in skips in the detection of some lesions. Ongoing improvements in screening techniques are likely to increase cervical cancer detection rates and decrease mortality rates.In 2021, the WHO Collaborating Centre for Cervical Cancer Elimination partnered with the University of Miami's Sylvester Comprehensive Cancer Centre, which will serve as a hub for research and technical assistance to help countries eliminate cervical cancer.
Thus, such technological advancements are likely to bring new trends in the CIN & HR-HPV market in the coming years.
Asia Pacific CIN & HR-HPV Treatment Market Overview
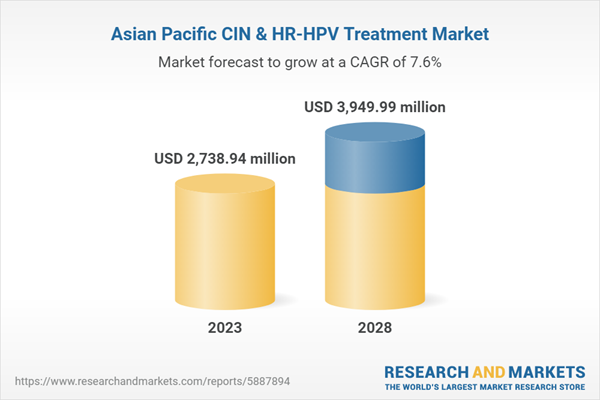
The Asia Pacific CIN & HR-HPV treatment market is segmented into China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific. China held the largest share of the market in 2022, and India is expected to exhibit a significant growth pace in the market. The market growth in these countries is attributed to the rising cervical cancer prevalence and HPV cases. Moreover, expansion and product launch by market players and various initiatives by government for cancer and HPV screening are expected to contribute to the growth of the CIN & HR-HPV treatment market in the region.Asia Pacific CIN & HR-HPV Treatment Market Revenue and Forecast to 2028 (US$ Million)
Asia Pacific CIN & HR-HPV Treatment Market Segmentation
The Asia Pacific CIN & HR-HPV treatment market is segmented into disease type, strain type, offering, product type, end user, and country.Based on disease type, the market is segmented into cervical intraepithelial neoplasia 1 (CIN 1), cervical intraepithelial neoplasia 2 (CIN 2), and cervical intraepithelial neoplasia 3 (CIN 3). The cervical intraepithelial neoplasia 3 (CIN 3) segment registered the largest market share in 2023.
Based on strain type, the market is segmented into HPV 16, HPV 18, and others. The HPV 16 segment held the largest market share in 2023.
Based on offering, the market is bifurcated into diagnostic methods and treatments. The treatments segment held a larger market share in 2023. Further, diagnostic methods are segmented into pap smear, HPV testing, colposcopy, and biopsy. Further, treatments are bifurcated into excision surgery and ablation techniques.
Based on product type, the Asia Pacific CIN & HR-HPV treatment market is segmented into kits & reagents, instruments, and services. The services segment held the largest market share in 2023.
Based on end user, the market is segmented into hospitals & clinics, diagnostic laboratories, specialized clinical laboratories, and others. The hospitals & clinics segment held the largest market share in 2023.
Based on country, the Asia Pacific CIN & HR-HPV treatment market is segmented into Australia, China, India, Japan, South Korea, and the Rest of Asia Pacific. China dominated the market share in 2023.
Fujirebio Europe NV; Qiagen NV; Abbott Laboratories; F. Hoffmann-LA Roche Ltd; Bioneer Corp ; and Thermo Fisher Scientific Inc are the leading companies operating in the Asia Pacific CIN & HR-HPV treatment market in the region.
Table of Contents
Companies Mentioned
- Fujirebio Europe NV
- Qiagen NV
- Abbott Laboratories
- F. Hoffmann-LA Roche Ltd
- Bioneer Corp
- Thermo Fisher Scientific Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 115 |
| Published | June 2023 |
| Forecast Period | 2023 - 2028 |
| Estimated Market Value ( USD | $ 2738.94 million |
| Forecasted Market Value ( USD | $ 3949.99 million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Asia Pacific |
| No. of Companies Mentioned | 6 |