Increase in Prevalence of Human Papillomavirus Infections Fuels Europe CIN & HR-HPV Treatment Market
Cervical intraepithelial neoplasia (CIN), also called cervical dysplasia, is characterized by the formation of proliferative lesions with abnormal cell growth on the surface of the cervix. These lesions replace a part of or the entire cervical squamous epithelium, and they are perceived as precursors of cervical cancer. Cervical dysplasia is caused by human papillomavirus (HPV) infection. Over 200 variants of HPV are known to cause infections among humans, and ~40 of these variants affect genitals upon spreading through sexual contact. 12 of these HPV types are associated with a high risk of cancer, and ~10 are associated with low risk cancer. ~70% of cervical cancer cases are caused by HPV type 16 and type 18, which are high-risk HPV (HR-HPV) serotypes. According to a study published in the National Library of Medicine, in 2022, most cervical cancer cases were caused by HPV 16, accounting for 55-60% of cervical cancer cases reported across the region in that year. Moreover, HPV 18 accounts for 10-15% of cervical cancer cases in the world. A persistent HPV infection can result in CIN among subjects that are immunocompromised and infected with HIV and have a history of smoking. According to the European Cancer Organization, in 2020, HPV infections were the cause of ~2.5% of cancer cases in Europe, and the prevalence of high-risk HPV infection exceeds 15% among the European population. According to the World Health Organization, ~66,000 women were diagnosed with cervical cancer in the WHO European region in 2022.Thus, a rise in the prevalence of HPV infections contributes to the growth of the Europe CIN & HR-HPV treatment market.
Europe CIN & HR-HPV Treatment Market Overview
The Europe CIN & HR-HPV treatment market is divided into Germany, the UK, France, Italy, Spain, and the Rest of Europe. The region holds a market share of the Europe CIN & HR-HPV treatment market, and it is estimated to register a robust growth rate during the forecast period. The growth of the CIN & HR-HPV treatment market in Europe is attributed due to the high incidence of cervical cancer and the increase in cervical cancer screening programs
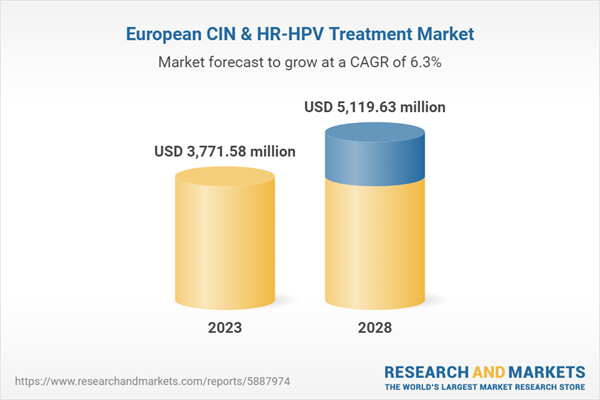
Exhibit: Europe CIN & HR-HPV Treatment Market Revenue and Forecast to 2028 (US$ Million)
Europe CIN & HR-HPV Treatment Market Segmentation
The Europe CIN & HR-HPV treatment market is segmented into disease type, strain type, offering, product type, end user, and country.Based on disease type, the market is segmented into cervical intraepithelial neoplasia 1 (CIN 1), cervical intraepithelial neoplasia 2 (CIN 2), and cervical intraepithelial neoplasia 3 (CIN 3). The cervical intraepithelial neoplasia 3 (CIN 3) segment registered the largest market share in 2023.
Based on strain type, the Europe CIN & HR-HPV treatment market is segmented into HPV 16, HPV 18, and others. The HPV 16 segment held the largest market share in 2023.
Based on offering, the Europe CIN & HR-HPV treatment market is bifurcated into diagnostic methods and treatments. The treatments segment held a larger market share in 2023. Further, diagnostic methods are segmented into pap smear, HPV testing, colposcopy, and biopsy. Further, treatments are bifurcated into excision surgery and ablation techniques.
Based on product type, the market is segmented into kits & reagents, instruments, and services. The services segment held the largest market share in 2023.
Based on end user, the Europe CIN & HR-HPV treatment market is segmented into hospitals & clinics, diagnostic laboratories, specialized clinical laboratories, and others. The hospitals & clinics segment held the largest market share in 2023.
Based on country, the Europe CIN & HR-HPV treatment market is segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. Germany dominated the market share in 2022.
Fujirebio Europe NV; Qiagen NV; Zilico Ltd; Abbott Laboratories; F. Hoffmann-LA Roche Ltd; Bioneer Corp; and Thermo Fisher Scientific Inc are the leading companies operating in the CIN & HR-HPV treatment market in the region.
Table of Contents
Companies Mentioned
- Fujirebio Europe NV
- Qiagen NV
- Zilico Ltd
- Abbott Laboratories
- F. Hoffmann-LA Roche Ltd
- Bioneer Corp
- Thermo Fisher Scientific Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 117 |
| Published | June 2023 |
| Forecast Period | 2023 - 2028 |
| Estimated Market Value ( USD | $ 3771.58 million |
| Forecasted Market Value ( USD | $ 5119.63 million |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 7 |