1. Research Methodology
1.1. Study Objectives
1.2. Study Scope
1.3. Research Assumptions
1.4. Research Framework
2. Introduction
2.1. Market Definition
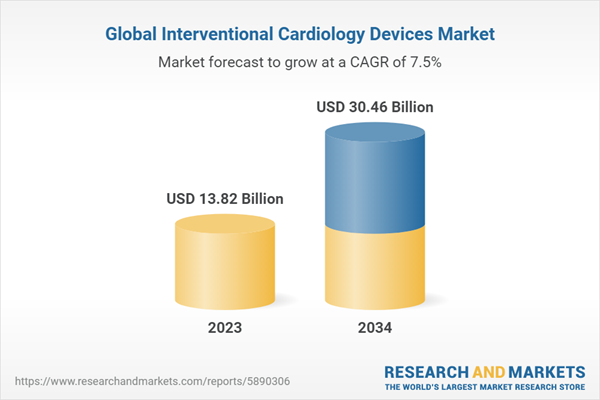
2.2. Global Interventional Cardiology Devices Market Overview
4. Market Environment Analysis
4.1. Porter’s 5 Forces Analysis
4.2. PESTEL Analysis
4.3. SWOT Analysis
5. Market Dynamics
5.1. Drivers Analysis
5.2. Restraints Analysis
5.3. Opportunities Analysis
5.4. Threats Analysis
5.5. Trend Analysis
7. Interventional Cardiology Devices Market: Product Estimates & Trend Analysis
7.1. Product Segment Opportunity Analysis
7.2. PTCA Balloon Catheters
7.2.1. PTCA Balloon Catheters Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.2. DCB
7.2.2.1. DCB Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.3. Normal
7.2.3.1. Normal Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.4. Specialty
7.2.4.1. Specialty Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.4.2. Cutting
7.2.4.2.1. Cutting Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.4.3. Scoring
7.2.4.3.1. Scoring Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.4.4. Lithotripsy Balloons
7.2.4.4.1. Lithotripsy Balloons Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3. Coronary Stents
7.3.1. Coronary Stents Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.2. Bioabsorbable Stents
7.3.2.1. Bioabsorbable Stents Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.3. Durg-eluting Stents
7.3.3.1. Durg-eluting Stents Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.4. Bare Stends
7.3.4.1. Bare Stends Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.4. Intravascular Imaging Catheters and Pressure Guidewires
7.4.1. Intravascular Imaging Catheters and Pressure Guidewires Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.4.2. OCT Catheters
7.4.2.1. OCT Catheters Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.4.3. IVUS Catheters
7.4.3.1. IVUS Catheters Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.4.4. FFR Guidewires
7.4.4.1. FFR Guidewires Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5. Accessory Devices
7.5.1. Accessory Devices Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5.2. PTCA Guiding Catheters
7.5.2.1. PTCA Guiding Catheters Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5.3. Diagnostic Catheters
7.5.3.1. Diagnostic Catheters Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5.4. Introducer Sheaths
7.5.4.1. Introducer Sheaths Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5.5. PTCA Guidewires
7.5.5.1. PTCA Guidewires Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8. Interventional Cardiology Devices Market: End-user Estimates & Trend Analysis
8.1. End-user Segment Opportunity Analysis
8.2. Hospitals & Ambulatory Surgical Centres
8.2.1. Hospitals & Ambulatory Surgical Centres Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3. Clinics & Others
8.3.1. Clinics & Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9. Regional Market Analysis
9.1. Regional Market Opportunity Analysis
10. North America Interventional Cardiology Devices Market
10.1. North America Interventional Cardiology Devices Market
10.1.1. North America Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
10.1.2. North America Interventional Cardiology Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
10.1.3. North America Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
10.1.4. North America Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
10.2. U.S. Global Interventional Cardiology Devices Market
10.2.1. U.S. Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
10.2.2. U.S. Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
10.2.3. U.S. Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
10.3. Canada Global Interventional Cardiology Devices Market
10.3.1. Canada Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
10.3.2. Canada Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
10.3.3. Canada Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11. Europe Global Interventional Cardiology Devices Market
11.1. Europe Global Interventional Cardiology Devices Market
11.1.1. Europe Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.1.2. Europe Interventional Cardiology Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
11.1.3. Europe Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.1.4. Europe Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.2. Germany Global Interventional Cardiology Devices Market
11.2.1. Germany Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.2.2. Germany Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.2.3. Germany Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.3. UK Global Interventional Cardiology Devices Market
11.3.1. UK Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.3.2. UK Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.3.3. UK Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.4. France Global Interventional Cardiology Devices Market
11.4.1. France Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.4.2. France Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.4.3. France Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.5. Spain Global Interventional Cardiology Devices Market
11.5.1. Spain Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.5.2. Spain Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.5.3. Spain Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.6. Italy Global Interventional Cardiology Devices Market
11.6.1. Italy Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.6.2. Italy Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.6.3. Italy Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.7. Rest of Europe Global Interventional Cardiology Devices Market
11.7.1. Rest of Europe Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.7.2. Rest of Europe Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.7.3. Rest of Europe Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12. Asia Pacific Global Interventional Cardiology Devices Market
12.1. Asia Pacific Global Interventional Cardiology Devices Market
12.1.1. Asia Pacific Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.1.2. Asia Pacific Interventional Cardiology Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
12.1.3. Asia Pacific Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.1.4. Asia Pacific Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.2. Japan Global Interventional Cardiology Devices Market
12.2.1. Japan Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.2.2. Japan Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.2.3. Japan Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.3. China Global Interventional Cardiology Devices Market
12.3.1. China Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.3.2. China Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.3.3. China Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.4. India Global Interventional Cardiology Devices Market
12.4.1. India Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.4.2. India Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.4.3. India Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.5. South Korea Global Interventional Cardiology Devices Market
12.5.1. South Korea Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.5.2. South Korea Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.5.3. South Korea Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.6. Australia Global Interventional Cardiology Devices Market
12.6.1. Australia Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.6.2. Australia Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.6.3. Australia Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.7. Rest of Asia Pacific Global Interventional Cardiology Devices Market
12.7.1. Rest of Asia Pacific Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.7.2. Rest of Asia Pacific Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.7.3. Rest of Asia Pacific Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13. Latin America Global Interventional Cardiology Devices Market
13.1. Latin America Global Interventional Cardiology Devices Market
13.1.1. Latin America Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.1.2. Latin America Interventional Cardiology Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
13.1.3. Latin America Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.1.4. Latin America Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.2. Brazil Global Interventional Cardiology Devices Market
13.2.1. Brazil Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.2.2. Brazil Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.2.3. Brazil Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.3. Mexico Global Interventional Cardiology Devices Market
13.3.1. Mexico Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.3.2. Mexico Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.3.3. Mexico Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.4. Argentina Global Interventional Cardiology Devices Market
13.4.1. Argentina Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.4.2. Argentina Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.4.3. Argentina Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.5. Rest of Latin America Global Interventional Cardiology Devices Market
13.5.1. Rest of Latin America Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.5.2. Rest of Latin America Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.5.3. Rest of Latin America Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14. MEA Global Interventional Cardiology Devices Market
14.1. MEA Global Interventional Cardiology Devices Market
14.1.1. MEA Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.1.2. MEA Interventional Cardiology Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
14.1.3. MEA Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.1.4. MEA Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.2. GCC Global Interventional Cardiology Devices Market
14.2.1. GCC Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.2.2. GCC Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.2.3. GCC Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.3. South Africa Global Interventional Cardiology Devices Market
14.3.1. South Africa Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.3.2. South Africa Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.3.3. South Africa Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.4. Rest of MEA Global Interventional Cardiology Devices Market
14.4.1. Rest of MEA Interventional Cardiology Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.4.2. Rest of MEA Interventional Cardiology Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.4.3. Rest of MEA Interventional Cardiology Devices Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15. Competitor Analysis
15.1. Company Market Share Analysis, 2023
15.2. Major Recent Developments
16. Company Profiles
16.1. Philips Healthcare
16.2. Medtronic
16.3. Biosensors International
16.4. Cardinal Health
16.5. BIOTRONIK
16.6. Terumo
16.7. Alvimedica
16.8. Cook Medical
16.9. Teleflex Medical
16.10. B. Braun
16.11. ASAHI INTECC
16.12. CID Vascular
16.13. Spectranetics
16.14. Boston Scientific
16.15. Abbott Laboratories
16.16. Meril Life Sciences
16.17. Vascular Solutions
16.18. NuMED
16.19. ACIST Medical Systems
16.20. Zeon Medical
16.21. Medinol
16.22. Merit Medical Systems
16.23. Opsens
16.24. Other Prominent Players