1. Research Methodology
1.1. Study Objectives
1.2. Study Scope
1.3. Research Assumptions
1.4. Research Framework
2. Introduction
2.1. Market Definition
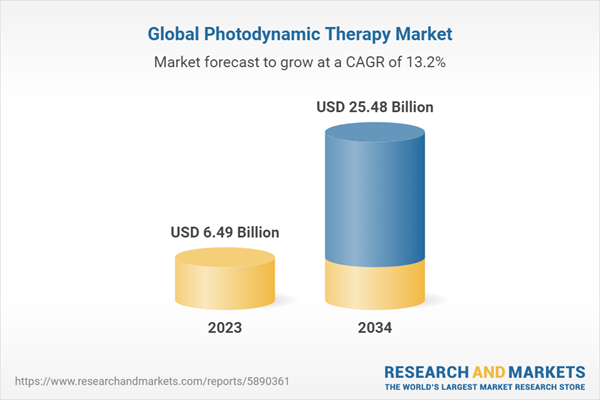
2.2. Global Photodynamic Therapy Market Overview
4. Market Environment Analysis
4.1. Porter’s 5 Forces Analysis
4.2. PESTEL Analysis
4.3. SWOT Analysis
5. Market Dynamics
5.1. Drivers Analysis
5.2. Restraints Analysis
5.3. Opportunities Analysis
5.4. Threats Analysis
5.5. Trend Analysis
7. Photodynamic Therapy Market: Product Estimates & Trend Analysis
7.1. Product Segment Opportunity Analysis
7.2. Photodynamic Therapy Devices
7.2.1. Photodynamic Therapy Devices Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.2. Disposable Fiber Optic Light Delivery Devices
7.2.2.1. Disposable Fiber Optic Light Delivery Devices Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.2.3. Diode Lasers
7.2.3.1. Diode Lasers Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3. Photosensitizer Drugs
7.3.1. Photosensitizer Drugs Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.2. Porphyrin Derivatives
7.3.2.1. Porphyrin Derivatives Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.3. Aminolevulinic Acid
7.3.3.1. Aminolevulinic Acid Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.4. Chlorines
7.3.4.1. Chlorines Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.5. Others
7.3.5.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8. Photodynamic Therapy Market: Application Estimates & Trend Analysis
8.1. Application Segment Opportunity Analysis
8.2. Actinic Keratosis
8.2.1. Actinic Keratosis Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3. Psoriasis
8.3.1. Psoriasis Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.4. Acne
8.4.1. Acne Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.5. Cancer
8.5.1. Cancer Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.6. Others
8.6.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9. Photodynamic Therapy Market: End-user Estimates & Trend Analysis
9.1. End-user Segment Opportunity Analysis
9.2. Cosmetic & Dermatology Clinics
9.2.1. Cosmetic & Dermatology Clinics Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.3. Hospitals
9.3.1. Hospitals Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.4. Others
9.4.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10. Regional Market Analysis
10.1. Regional Market Opportunity Analysis
11. North America Photodynamic Therapy Market
11.1. North America Photodynamic Therapy Market
11.1.1. North America Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.1.2. North America Photodynamic Therapy Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
11.1.3. North America Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.1.4. North America Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
11.1.5. North America Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.2. U.S. Global Photodynamic Therapy Market
11.2.1. U.S. Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.2.2. U.S. Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.2.3. U.S. Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
11.2.4. U.S. Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
11.3. Canada Global Photodynamic Therapy Market
11.3.1. Canada Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
11.3.2. Canada Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
11.3.3. Canada Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
11.3.4. Canada Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12. Europe Global Photodynamic Therapy Market
12.1. Europe Global Photodynamic Therapy Market
12.1.1. Europe Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.1.2. Europe Photodynamic Therapy Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
12.1.3. Europe Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.1.4. Europe Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.1.5. Europe Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.2. Germany Global Photodynamic Therapy Market
12.2.1. Germany Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.2.2. Germany Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.2.3. Germany Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.2.4. Germany Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.3. UK Global Photodynamic Therapy Market
12.3.1. UK Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.3.2. UK Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.3.3. UK Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.3.4. UK Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.4. France Global Photodynamic Therapy Market
12.4.1. France Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.4.2. France Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.4.3. France Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.4.4. France Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.5. Spain Global Photodynamic Therapy Market
12.5.1. Spain Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.5.2. Spain Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.5.3. Spain Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.5.4. Spain Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.6. Italy Global Photodynamic Therapy Market
12.6.1. Italy Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.6.2. Italy Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.6.3. Italy Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.6.4. Italy Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
12.7. Rest of Europe Global Photodynamic Therapy Market
12.7.1. Rest of Europe Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.7.2. Rest of Europe Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.7.3. Rest of Europe Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.7.4. Rest of Europe Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13. Asia Pacific Global Photodynamic Therapy Market
13.1. Asia Pacific Global Photodynamic Therapy Market
13.1.1. Asia Pacific Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.1.2. Asia Pacific Photodynamic Therapy Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
13.1.3. Asia Pacific Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.1.4. Asia Pacific Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.1.5. Asia Pacific Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.2. Japan Global Photodynamic Therapy Market
13.2.1. Japan Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.2.2. Japan Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.2.3. Japan Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.2.4. Japan Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.3. China Global Photodynamic Therapy Market
13.3.1. China Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.3.2. China Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.3.3. China Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.3.4. China Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.4. India Global Photodynamic Therapy Market
13.4.1. India Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.4.2. India Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.4.3. India Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.4.4. India Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.5. South Korea Global Photodynamic Therapy Market
13.5.1. South Korea Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.5.2. South Korea Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.5.3. South Korea Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.5.4. South Korea Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.6. Australia Global Photodynamic Therapy Market
13.6.1. Australia Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.6.2. Australia Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.6.3. Australia Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.6.4. Australia Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.7. Rest of Asia Pacific Global Photodynamic Therapy Market
13.7.1. Rest of Asia Pacific Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.7.2. Rest of Asia Pacific Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.7.3. Rest of Asia Pacific Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.7.4. Rest of Asia Pacific Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14. Latin America Global Photodynamic Therapy Market
14.1. Latin America Global Photodynamic Therapy Market
14.1.1. Latin America Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.1.2. Latin America Photodynamic Therapy Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
14.1.3. Latin America Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.1.4. Latin America Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.1.5. Latin America Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.2. Brazil Global Photodynamic Therapy Market
14.2.1. Brazil Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.2.2. Brazil Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.2.3. Brazil Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.2.4. Brazil Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.3. Mexico Global Photodynamic Therapy Market
14.3.1. Mexico Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.3.2. Mexico Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.3.3. Mexico Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.3.4. Mexico Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.4. Argentina Global Photodynamic Therapy Market
14.4.1. Argentina Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.4.2. Argentina Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.4.3. Argentina Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.4.4. Argentina Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.5. Rest of Latin America Global Photodynamic Therapy Market
14.5.1. Rest of Latin America Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.5.2. Rest of Latin America Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.5.3. Rest of Latin America Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.5.4. Rest of Latin America Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15. MEA Global Photodynamic Therapy Market
15.1. MEA Global Photodynamic Therapy Market
15.1.1. MEA Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.1.2. MEA Photodynamic Therapy Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
15.1.3. MEA Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.1.4. MEA Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.1.5. MEA Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.2. GCC Global Photodynamic Therapy Market
15.2.1. GCC Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.2.2. GCC Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.2.3. GCC Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.2.4. GCC Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.3. South Africa Global Photodynamic Therapy Market
15.3.1. South Africa Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.3.2. South Africa Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.3.3. South Africa Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.3.4. South Africa Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.4. Rest of MEA Global Photodynamic Therapy Market
15.4.1. Rest of MEA Photodynamic Therapy Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.4.2. Rest of MEA Photodynamic Therapy Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.4.3. Rest of MEA Photodynamic Therapy Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.4.4. Rest of MEA Photodynamic Therapy Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
16. Competitor Analysis
16.1. Company Market Share Analysis, 2023
16.2. Major Recent Developments
17. Company Profiles
17.1. biolitec AG
17.2. Galderma S.A.
17.3. Biofrontera AG
17.4. Soligenix
17.5. Modulight, Inc.
17.6. Lumibird (Quantel Medical)
17.7. Theralase Technologies Inc.
17.8. Bausch Health Companies Inc.
17.9. Sun Pharmaceutical Industries Limited
17.10. Other Prominent Players