Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
This is due to the fact that the medicine's or pharmaceutical drug's patent has expired. Drugs that can be used to treat hypertension, diabetes, back pain, thyroid, and arthritis, among other conditions, are re-released at cheaper costs in order to stay in the market and survive competition. However, it is important to highlight that, despite the alterations made in the re-release of the drug, the quality remains the same. The functions that the medicine would accomplish are not compromised in any way.
Key Market Drivers
Increasing Prevalence of Chronic Diseases
The rising prevalence of chronic diseases is a major driving force behind the expansion of the Global Branded Generics Market. Approximately 9 million people succumb to cancer annually, while 4 million deaths are attributed to chronic respiratory diseases like asthma and COPD, and another 2 million result from diabetes-related complications. However, the global distribution of disease burden and access to advanced healthcare solutions remains highly uneven, with disparities particularly evident in low- and middle-income regions where medical infrastructure, early diagnostics, and treatment accessibility are significantly limited.With a growing global disease burden, healthcare systems and consumers are increasingly turning to cost-effective and reliable treatment options, making branded generics a preferred choice. Chronic diseases such as cardiovascular diseases (CVDs), diabetes, cancer, respiratory disorders, and arthritis are becoming more prevalent due to factors such as aging populations, unhealthy lifestyles, and environmental changes.
According to the World Health Organization (WHO), non-communicable diseases (NCDs) account for nearly 74% of all global deaths, highlighting the urgent need for long-term treatment solutions. Increased demand for continuous medication supply for long-term disease management. Greater emphasis on cost-effective treatment alternatives, making branded generics a viable solution. Higher healthcare spending by governments and private insurers to address chronic disease care.
Key Market Challenges
Regulatory Hurdles
Generic medications, including branded generics, need to go through regulatory approval processes to ensure their safety, quality, and efficacy. Delays or complexities in the approval process can slow down the introduction of new branded generic products to the market. Regulatory authorities often have strict requirements for demonstrating the bioequivalence of branded generics to their branded counterparts. Meeting these requirements can be challenging and time-consuming, potentially causing delays in market entry. Regulatory challenges can arise when dealing with the patents of the original branded drugs. Patent disputes and legal battles can delay or prevent the launch of branded generics even after the patent has expired.Different countries have varying regulatory frameworks and requirements for generic medications. Navigating these diverse regulations can be complex for pharmaceutical companies looking to market branded generics globally. Ensuring consistent quality and adherence to manufacturing standards is crucial for the acceptance of generic medications. Regulatory hurdles related to quality control can affect the perception and trust of healthcare professionals and patients in branded generics. Some regulatory systems offer periods of data exclusivity or market exclusivity for innovator drugs, which can delay the entry of generic alternatives. This can hinder the availability of branded generics, especially in markets with longer exclusivity periods.
Key Market Trends
Advancements in Digital Health Integration
Digital health technologies can improve patient engagement by providing tools for medication reminders, tracking, and adherence. Branded generics, as more affordable alternatives, can benefit from increased patient engagement, leading to improved treatment outcomes and patient loyalty. Branded generics are often used for chronic disease management. Digital health solutions enable remote monitoring of patients' health conditions, which is particularly useful for managing chronic diseases. This integration can enhance patient compliance with medication regimens, contributing to the growth of the market.Digital health platforms can facilitate telemedicine consultations, where physicians can remotely diagnose and prescribe medications, including branded generics. This can increase the accessibility of these medications to patients, especially in regions with limited healthcare infrastructure. Digital health solutions can enable personalized treatment plans based on patient data and health metrics. Branded generics can be tailored to fit individual patient needs, resulting in better treatment outcomes and patient satisfaction.
Digital health integration can improve awareness of branded generics among patients and healthcare providers. Through digital platforms, manufacturers can educate users about the benefits, availability, and cost savings associated with branded generics. Pharmaceutical companies can use data analytics to gain insights into market trends, patient preferences, and healthcare utilization patterns. This information can guide marketing strategies and product positioning for branded generics.
Key Market Players
- Teva Pharmaceutical Industries Ltd.
- Lupin Pharmaceuticals Inc.
- Sanofi-Aventis.
- Sun Pharmaceutical Industries Inc.
- Dr Reddy's Laboratories Inc.
- Endo International PLC.
- GlaxoSmithKline LLC.
- Pfizer Inc.
- Viatris Inc.
- Apotex Inc.
Report Scope:
In this report, the Global Branded Generics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Branded Generics Market, By Drug Class:
- Alkylating Agents
- Antimetabolites
- Hormones
- Anti - Hypertensive
- Lipid Lowering Drugs
- Antidepressant
- Antipsychotics
- Antiepileptics
- Others
Branded Generics Market, By Application:
- Oncology
- Cardiovascular Diseases
- Neurological Diseases
- Acute & Chronic Pain
- Gastrointestinal Diseases
- Dermatological Diseases
- Others
Branded Generics Market, By Distribution Channel:
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Branded Generics Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Branded Generics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Teva Pharmaceutical Industries Ltd.
- Lupin Pharmaceuticals Inc.
- Sanofi-Aventis.
- Sun Pharmaceutical Industries Inc.
- Dr Reddy's Laboratories Inc.
- Endo International PLC.
- GlaxoSmithKline LLC.
- Pfizer Inc.
- Viatris Inc.
- Apotex Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
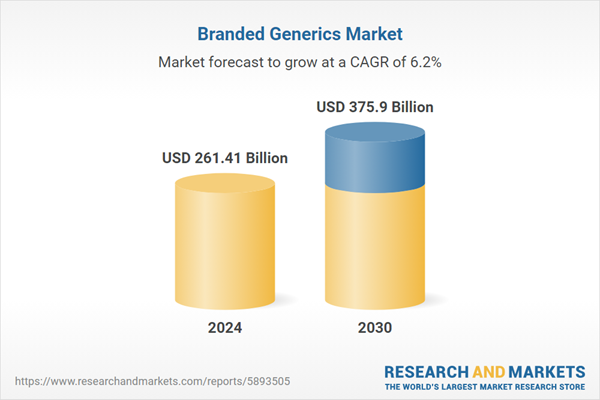
| Estimated Market Value ( USD | $ 261.41 Billion |
| Forecasted Market Value ( USD | $ 375.9 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |