Wearable Pregnancy Devices: Introduction
Wearable pregnancy devices are innovative wearable technologies, designed to provide monitoring, tracking, and information related to pregnancy and maternal health. These devices are designed to be worn by pregnant individuals, offering real-time data collection and insights that can help monitor the well-being of both the mother and the developing baby. Different type of wearable pregnancy devices used for a wide range of purposes include pregnancy trackers, fetal doppler monitors, maternal health monitors, contractions monitors, kick counters, temperature sensors, remote monitoring systems, smart clothing, nutrition and hydration trackers, labor monitors.Global Wearable Pregnancy Devices Market Analysis
The increasing research and development activities are primarily responsible for the market growth. The increasing investment by key players and others is also a major trend influencing the market growth as development of such devices is important to improve the patient outcome among pregnant women facing difficulties related to pregnancy.Additionally, the university of Melbourne has announced a seed pre-seed investment in support to two innovative female-founded healthcare startups for developing an innovative at-home pregnancy monitoring start-up Kali Healthcare, and clinical trial participant recruitment start-up Torch Recruit.
A small wearable device and sensor patch designed to observe baby's heart rate accurately has been developed by Kali Healthcare, co-founded by University of Melbourne Associate Professor Fiona Brownfoot. This device is anticipated to improve the access to pregnancy monitoring and make the whole process a lot easier.
The increased investments by government and initiatives, such as awareness campaigns to create awareness among people about these devices and their advantages, is driving the global wearable pregnancy devices market growth. The rising demand for wearable pregnancy devices is due to the reliability provided which is backed by the research. These medical-oriented devices that are approved to continuously monitor patients and their daily activities are expected to further propel the market growth.
Global Wearable Pregnancy Devices Market Segmentations
Wearable Pregnancy Devices Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Products
- Heart Rate Monitoring Devices
- Blood Pressure Monitoring Devices
- Real Time Contraction Tracking Devices
- Health Tracking Devices
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Market Breakup by End User
- Hospitals and Clinics
- Ambulatory Surgical Centers
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Wearable Pregnancy Devices Market Overview
The market for wearable pregnancy products is expected to grow due to the increasing awareness of fitness and lifestyle improvements among pregnant women across the globe. The convenient usage of these wearable pregnancy devices and the minimum effort required to set them up is also a major cause of the increased demand of these devices in the market. The increasing technological advancements in pregnancy gadgets are also driving the global wearable pregnancy devices market growth.The rising product launches by key players in the market is also driving the market growth. The rising disposable income among people is also a major factor driving the market growth as it enables the users to acquire these devices that could be a bit unaffordable for people without disposable incomes. Provided all the above factors, the lack of awareness about the advantages of using wearable pregnancy devices in countries still developing is restricting the market growth in certain countries.
Wearable Pregnancy Devices Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Koninklijke Philips N.V.
- Bloomlife
- NUVO Inc.
- Bellabeat
- Abbott
- Medtronic
- Brainlab AG
- Varian Medical Systems, Inc.
- Hitachi
- Biotricity
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Koninklijke Philips N.V.
- Bloomlife
- NUVO Inc.
- Bellabeat
- Abbott
- Medtronic
- Brainlab AG
- Varian Medical Systems, Inc.
- Hitachi
- Biotricity
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
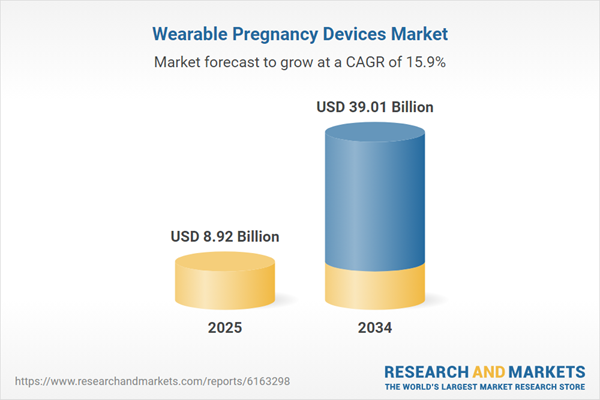
| Estimated Market Value ( USD | $ 8.92 Billion |
| Forecasted Market Value ( USD | $ 39.01 Billion |
| Compound Annual Growth Rate | 15.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |