Secondary Myelofibrosis Therapeutics: Introduction
Myelofibrosis is a rare type of blood cancer where bone marrow (the soft, spongy tissue inside of your bones) is replaced by fibrous scar tissue. It is a form of chronic leukemia and a myeloproliferative disorder. Myeloproliferative disorders involve too many blood cells getting made in bone marrow- where blood cells get made.Bone marrow produces immature blood-forming cells called stem cells that may develop into red blood cells, white blood cells or platelets. With myelofibrosis, a change (mutation) in a stem cell's DNA causes the cell to become defective, or a cancer cell, instead. The cell multiplies, passing the mutation onto new cells. There are two types of myelofibrosis:
- Primary myelofibrosis is myelofibrosis that occurs on its own.
- Secondary myelofibrosis arises secondary to other blood disorders, including primary thrombocytosis or polycythemia vera. Secondary myelofibrosis accounts for about 10% to 20% of diagnoses.
Global Secondary Myelofibrosis Therapeutics Market Analysis
The continuous technological advancement and development of new therapies are the key factors driving the market growth. The increasing investments by key players and government in research and development of new therapies are also driving the market growth. Disorders that involve the overproduction of certain blood cell types leading to changes in the bone marrow such as polycythemia vera, essential thrombocythemia. The increasing emphasis on research and development of new treatments to overcome the condition in patients is a major trend influencing the global secondary myelofibrosis therapeutics market expansion. To overcome the issues associated with early JAK inhibitors (ruxolitinib and fedratinib), such as limited tolerability, pacritinib has been developed and recently approved for patients with thrombocytopenia, while momelotinib is in development for those with anemia. Pacritinib is an anti-cancer medication used to treat myelofibrosis and momelotinib is an inhibitor of Janus kina.The key players are also continuously putting efforts towards developing the most effective drug and the rising approvals, which is likely to add further value to the global secondary myelofibrosis therapeutics market share. For example, Karyopharm Therapeutics Inc., a commercial-stage pharmaceutical company pioneering novel cancer therapies, made it public about the Fast Track Designation grant put in action by the United States Food and Drug Administration (FDA) to the development program of Selinexor. It will be used in the treatment of myelofibrosis, including primary myelofibrosis, post-essential thrombocythemia myelofibrosis, and post-polycythemia vera myelofibrosis.
Additionally, the rising number of clinical trials are also increasing the awareness and curiosity of healthcare providers and researchers to explore the best potential drug to treat secondary myelofibrosis, leading to the market growth. For instance- the results of a case study on SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM trials exhibited improvements in symptom score and spleen reduction. It also suggested a possible treatment for MF-related anemia. A decrease in overall transfusions and an increase in transfusion independence was witnessed in all three trials.
Global Secondary Myelofibrosis Therapeutics Market Segmentations
The report titled “ Secondary Myelofibrosis Therapeutics Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market breakup Market by Type
- Targeted Therapy
- Chemotherapy
- Radiation Therapy
Market breakup by Diagnosis
- Physical Tests
- Imaging Tests
- Blood Tests
Market breakup by Drug Class
- Ruxolitnab
- Fedratinib
- Pomalidomide
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Secondary Myelofibrosis Therapeutics Market Overview
The increasing prevalence of cancer is also a major factor contributing to the market growth as cancer treatments, such as chemotherapy and radiation therapy, can damage bone marrow cells, which eventually may result in the development of myelofibrosis. The increasing development of drugs and increasing awareness about combination therapies are also contributing to the global secondary myelofibrosis therapeutics market development such as multiple drugs are currently being developed and examined in clinical trials, both as standalone therapy and in combination with JAK inhibitors, with promising results increasing the benefits of JAK inhibitors in treatment of myelofibrosis.The rising awareness about cancer treatments such as chemotherapy and radiotherapy are also driving the market growth. The increasing advancements in therapeutic treatment approaches for secondary myelofibrosis are further propelling the market growth. Along with above mentioned factors, large patient pool, increasing incidences of genetic disorders, rising habit of smoking are also continuously increasing the global secondary myelofibrosis therapeutics market growth.
Geographically, North America is expected to lead the regional market due to the rising prevalence of secondary myelofibrosis, rising advancements. Ongoing research and development activities along with approval of JAK inhibitors by FDA due to its efficacy shown by the treatment is a major factor driving the market growth. The well-established healthcare system of North America is also expected to drive growth due to the presence of robust advanced technologies for the treatment.
Secondary Myelofibrosis Therapeutics Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Competition Deep Dive
- Incyte Corporation
- Bristol-Myers Squibb Company
- Amneal Pharmaceuticals, Inc.
- AbbVie Inc.
- GlaxoSmithKline plc
- Pfizer Inc.
- Actuate Therapeutics Inc.
- Imago Biosciences
- Galecto, Inc
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Competition Deep Dive
- Incyte Corporation
- Bristol-Myers Squibb Company
- Amneal Pharmaceuticals, Inc.
- AbbVie Inc.
- GlaxoSmithKline plc
- Pfizer Inc.
- Actuate Therapeutics Inc.
- Imago Biosciences
- Galecto, Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
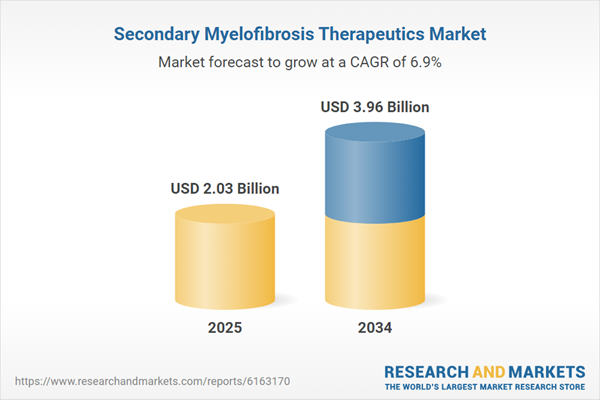
| Estimated Market Value ( USD | $ 2.03 Billion |
| Forecasted Market Value ( USD | $ 3.96 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |