Lateral Flow Assay: Introduction
Lateral flow assay is a diagnostic device used to detect and quantify analyte (pathogen, antibody, or biomarker) in a human or animal. It is commonly used in COVID-19 antibody rapid tests, Pregnancy (HCG hormone) tests, HIV tests, and Hepatitis B virus (HBV) tests. Hospitals and clinical labs widely rely on lateral flow assays as they are easy to use, portable, available at affordable process and provide immediate results. It shows qualitative test results via visual inspection under 30 minutes.There are two types of lateral flow assays, categorised on the types of molecules to be identified. First is the non-competitive or sandwich test, used for macromolecular analytes (substance to analysed in a sample). It has two antigen binding sites and is commonly used in pregnancy tests. Second type is a competitive test, which is mostly used for drug tests and has less than two antigen binding sites.
Global Lateral Flow Assays Market Analysis
With the COVID-19 outbreak, the concept of rapid tests has gained acceptance with the common people. With the aim to inhibit further contamination, governments have also worked to spread awareness and promote using rapid tests at home itself. Hence, the market for lateral flow assays has increased exponentially in a very short span of time. With a high demand, healthcare companies have also brought significant changes to the pre-existing models to make it more user-friendly and accurate.The world human population reached 8 billion in November 2023. It is estimated to increase by another 2 billion in the next 30 years and hit approximately 9.7 billion in 2050s . As the population keeps growing at a steady pace, pregnancy cases are also rising. With this the global market for lateral flow assays is observing a surge with the escalated demand of pregnancy detection kits.
Apart from clinical and drug testing, lateral flow assays are also relevant in food safety and environment testing. It is used to detect various food pathogens, drug residues, food additives and other illegal additives, etc. With a greater emphasis on consuming unadulterated and contamination-free food, the lateral flow assay market development can be expected in the food industry as well.
Global Lateral Flow Assays Market Segmentation
Lateral Flow Assays Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Kits and Reagents
- Lateral Flow Readers
Market Breakup by Technique
- Sandwich Assays
- Competitive Assays
- Multiplex Detection Assays
Market Breakup by Application
- Clinical Testing
- Veterinary Diagnostics
- Food Safety and Environment Testing
- Drug Development and Quality Testing
Market Breakup by End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Home Care
- Pharmaceutical and Biotechnology Companies
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Lateral Flow Assays Market Overview
Conventional lateral flow assay mechanism has shown remarkable performance in pregnancy tests and for COVID-19 tests, owing to its simplicity, promptness, and visual representation in the results. Yet, researchers and scientists are on the look out to bring an improved version of the detection kits. The results are measured by the colour changes and are easily readable by the naked eyes. However, they lack the qualitative analysis and sensitivity, which might hinder its use in other applications. To combat this, there has been further development of new nanomaterials to enhance LFA sensitivity. With ongoing advancements, the global lateral flow assays market share will expand further in the coming years.In the historical period, most of the market share has been dominated by the North American territory, credits to their impressive investments in the healthcare industry. But a market shift can be anticipated towards the Asian region. This is because the Asian region is likely to experience the greatest increase in the number of pregnancies in the following years. As a result, demand for pregnancy kits will be higher.
Global Lateral Flow Assays Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Thermo Fisher Scientific, Inc.
- Abbott
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche AG
- Quidel Corporation
- bioMérieux SA
- PerkinElmer, Inc.
- Hologic, Inc.
- Merck KGaA
- Siemens Healthineers
- Danaher Corporation
- QIAGEN N.V.
- BD
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific, Inc.
- Abbott
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche AG
- Quidel Corporation
- bioMérieux SA
- PerkinElmer, Inc.
- Hologic, Inc.
- Merck KGaA
- Siemens Healthineers
- Danaher Corporation
- QIAGEN N.V.
- BD
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
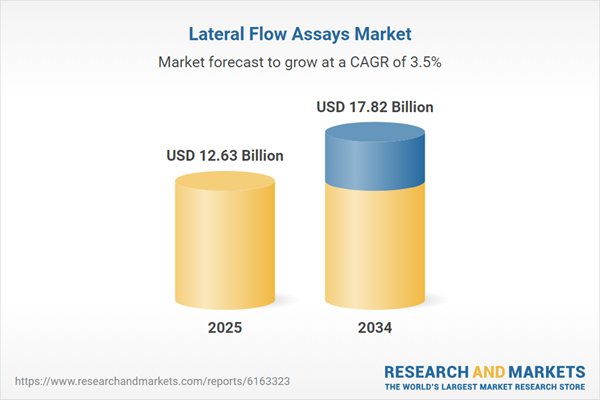
| Estimated Market Value ( USD | $ 12.63 Billion |
| Forecasted Market Value ( USD | $ 17.82 Billion |
| Compound Annual Growth Rate | 3.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |