Transcatheter Heart Valve Replacement and Repair: Introduction
Transcatheter aortic valve replacement and repair are procedures done to treat a stiff (calcified) or thickened aortic valve that cannot fully open (aortic stenosis). The aortic valve is located between left ventricle (lower heart chamber) and aorta (body's main artery). If this valve does not open properly, blood flow from heart to other parts is compromised and the heart is forced to work harder.Transcatheter aortic valve replacement (TAVR) is a minimally invasive procedure that helps avoid the need for open heart surgery in a patient. TAVR can help a patient live a prolonged and comfortable life by restoring blood flow and reducing the symptoms of aortic valve stenosis like chest pain (angina) or tightness, shortness of breath, fluttering heartbeat (palpitations), an irregular heart sound (heart murmur) heard through a stethoscope, fainting and fatigue. Transcatheter aortic valve replacement (TAVR) is also called transcatheter aortic valve implantation (TAVI).
Global Transcatheter Heart Valve Replacement and Repair Market Analysis
Congenital heart defect is a major cause of aortic valve stenosis. It is the most common congenital disorder, responsible for about 28% of all congenital birth defects. In this defect, children are born with an aortic valve that has only two cusps instead of three. Children born with this defect require regular medical checkups. It may not cause any problems until adulthood but if the valve begins to narrow or leak, it may need to be repaired or replaced. With approximately 1.35 million babies born with CHD each year globally, the transcatheter heart valve replacement and repair market has experienced a parallel growth, driven by the increasing number of patients necessitating treatment.Coronary artery calcification (CAC), which is highly prevalent in patients with coronary heart disease, grows with aging. By the time a person touches 70 or 80 years, calcium deposit starts on the valves. The stiffening valves need to be treated in time to avoid early demise. Hence, regions with increasing geriatric population are anticipated to observe a growth in the transcatheter heart valve replacement and repair market share.
Given the prevailing lifestyle characterized by obesity, high stress, blood pressure, and glucose levels, people are already at the risk of heart failures and open-heart surgeries. If such patterns persist, the prevalence of cardiovascular diseases is expected to escalate in the near future, potentially undergoing TAVR or TAVI to maintain a healthy life. As a result, there is a projected global expansion in the market size for transcatheter heart valve replacement and repair.
Global Transcatheter Heart Valve Replacement and Repair Market Segmentation
Transcatheter Heart Valve Replacement and Repair Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Procedure
- Transcatheter Aortic Valve Replacement (TAVR)
- Transcatheter Mitral Valve Repair (TMVR)
- Transcatheter Pulmonary Valve Replacement (TPVR)
Market Breakup by Indication
- Aortic Stenosis
- Mitral Regurgitation
- Aortic Regurgitation
- Mitral Stenosis
- Tricuspid Regurgitation
Market Breakup by End User
- Hospitals
- Cardiac Catheterization Laboratories
- Ambulatory Surgical Centres
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Transcatheter Heart Valve Replacement and Repair Market Overview
There have been significant advancements in the field of cardiac sciences, despite the high prevalence of patients with some or the other form of cardiac disease. Medtronic's next-generation Evolut FX system is refined with gold markers built into the frame of the implant to visualise the implant depth and location better. Abbott's Portico with FlexNav TAVI System requires 76% less insertion force than their previous products. It is evident that the healthcare industry is growing to develop high-quality and innovative techniques to facilitate the procedures to precision.Whether considering the highest number of cases or significant innovations, the North American region has been a focal point for the global transcatheter heart valve replacement and repair market development. Nonetheless, an anticipation of substantial demand is indicated towards the Asian region, primarily linked to its increasing emphasis on healthcare. The Asian territory constitutes to the maximum aging population globally. Hence, the prevalence of aortic stenosis and the cardiac surgical interventions are likely to increase, and the market size can expand in the future.
Global Transcatheter Heart Valve Replacement and Repair Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- Edwards Lifesciences Corporation
- Medtronic
- Abbott
- Boston Scientific Corporation
- B. Braun SE
- MicroPort Scientific Corporation
- Meril Life Sciences Pvt. Ltd.
- Sahajanand Medical Technologies Limited
- Venus Medtech (Hangzhou) Inc.
- Peijia Medical Limited
- Blue Sail Medical Co Ltd
- Penumbra, Inc.
- Encapson
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Edwards Lifesciences Corporation
- Medtronic
- Abbott
- Boston Scientific Corporation
- B. Braun SE
- MicroPort Scientific Corporation
- Meril Life Sciences Pvt. Ltd.
- Sahajanand Medical Technologies Limited
- Venus Medtech (Hangzhou) Inc.
- Peijia Medical Limited
- Blue Sail Medical Co Ltd
- Penumbra, Inc.
- Encapson
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
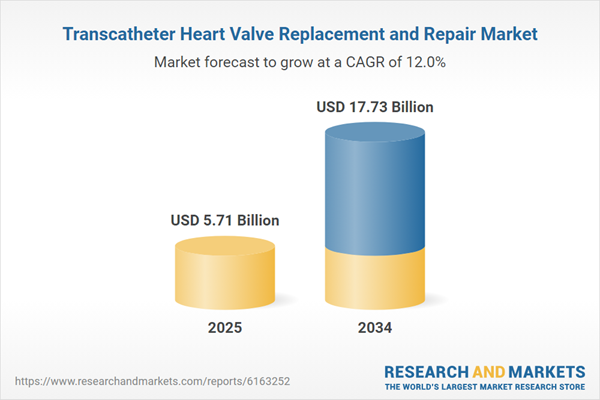
| Estimated Market Value ( USD | $ 5.71 Billion |
| Forecasted Market Value ( USD | $ 17.73 Billion |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |