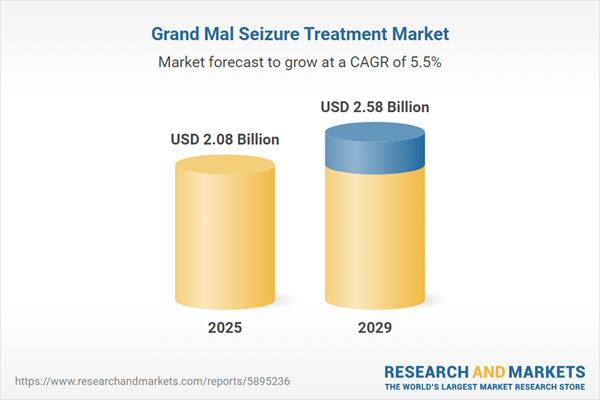
The grand mal seizure treatment market size is expected to see strong growth in the next few years. It will grow to $2.58 billion in 2029 at a compound annual growth rate (CAGR) of 5.5%. The growth in the forecast period can be attributed to genetic research and precision medicine, telemedicine for remote care, regulatory changes in accessibility, enhanced brain imaging techniques, holistic approach to treatment. Major trends in the forecast period include global advocacy for epilepsy awareness, patient-centered care models, expanded research in seizure genetics, community support networks, and non-pharmacological treatment approaches.
The forecast of 5.5% growth over the next five years reflects a slight reduction of 0.2% from the previous projection. This reduction is primarily due to the impact of tariffs between the US and other countries. The imposition of tariffs could disrupt U.S. epilepsy management by increasing prices of valproate and levetiracetam generics and electroencephalogram monitoring devices sourced from Israel and Ireland, delaying seizure control and raising neurology care burdens. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The anticipated growth of the grand mal seizure treatment market is strongly influenced by the high prevalence of epilepsy. Epilepsy, a neurological disorder characterized by recurring seizures, often manifests as grand mal seizures, which are particularly intense and violent. The rising prevalence of epilepsy worldwide underscores the pressing need for improved treatment options, specifically tailored to address grand mal seizures. As of February 2023, the World Health Organization reported that approximately 50 million people globally were grappling with epilepsy, marking it as one of the most prevalent neurological conditions globally. With an annual diagnosis rate of about 5 million people, the escalating prevalence of epilepsy serves as a primary driver fueling the growth of the grand mal seizure treatment market.
The growth trajectory of the grand mal seizure treatment market is further propelled by the increasing adoption of personalized medicines. Personalized medicine, an innovative healthcare approach, tailors medical treatments to the unique characteristics of individual patients. In the context of grand mal seizure treatment, personalized medicine involves analyzing an individual's genetic and molecular profile to customize interventions, such as selecting medications that are likely to be more effective and better tolerated based on the patient's unique characteristics. This precision-driven approach holds the potential to enhance seizure control while minimizing adverse effects for each patient. In 2022, the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) approved 37 new molecular entities (NMEs), and approximately 34% of these, amounting to 12 NMEs, were classified as personalized medicines by the Personalized Medicine Coalition (PMC). This significant adoption of personalized medicine practices is a key factor driving the growth of the grand mal seizure treatment market.
Major players in the grand mal seizure treatment market are strategically introducing new products, with a focus on anti-epilepsy medications, to gain a competitive advantage. Anti-epilepsy medications, also known as antiepileptic drugs (AEDs) or anticonvulsants, form a crucial class of pharmaceuticals designed to manage and treat epileptic seizures and related disorders. An illustrative example is Zydus Lifesciences Limited, an India-based pharmaceutical company, which, in January 2023, launched Topiramate extended-release capsules in the U.S. market. Zydus achieved the distinction of being the first company to receive final approval and introduce the product in strengths of USP 25 mg, 50 mg, and 100 mg. These capsules are indicated for epilepsy treatment, specifically as initial monotherapy for patients aged six and older with partial-onset or primary generalized tonic-clonic seizures. Additionally, they are approved for migraine prophylaxis in patients aged 12 and older, marking a significant contribution to the evolving landscape of grand mal seizure treatment.
Major companies actively participating in the grand mal seizure treatment market are advancing product development by introducing advanced offerings, particularly small molecule anticonvulsants for refractory focal onset seizures. Small molecule anticonvulsants, characterized by low molecular weight compounds, are instrumental in preventing or controlling seizures in individuals with epilepsy or other neurological disorders. An exemplar in this domain is Xenon Pharmaceuticals Inc., a Canada-based biopharmaceutical company, which launched the Phase 3 program for XEN1101 in November 2022 with the initiation of the X-TOLE2 clinical trial. This trial aims to investigate the use of XEN1101 as an adjunctive treatment for adult patients with focal epilepsy. XEN1101, a differentiated Kv7 potassium channel opener, has garnered positive Phase 2b clinical data, supporting its progression through late-stage clinical development toward commercialization. The introduction of advanced small molecule anticonvulsants represents a strategic move to establish a competitive edge in the dynamic grand mal seizure treatment market.
In March 2022, UCB, a biopharmaceutical company headquartered in Belgium, completed the acquisition of Zogenix for an estimated $1.9 billion. This strategic acquisition is poised to enable UCB to broaden its portfolio within emerging therapy segments and expand its presence in new geographical regions. Zogenix, based in the United States, specializes in the development and production of medications and therapies targeted at treating grand mal seizures. This acquisition signifies UCB's commitment to advancing its offerings in the treatment landscape for grand mal seizures and expanding its global footprint in the pharmaceutical industry.
Major companies operating in the grand mal seizure treatment market include Novartis AG, Sanofi S.A., GlaxoSmithKline PLC, Takeda Pharmaceuticals Inc., Mylan N.V., UCB Celltech Ltd., Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries Ltd., Aurobindo Pharma Limited, Lupin Pharmaceuticals Inc., Dr. Reddy's Laboratories Ltd., Cipla Inc., Apotex Inc., Hikma Pharmaceuticals PLC, Alkem Laboratories Ltd., Jubilant Life Sciences Limited, Torrent Pharmaceuticals Ltd., Zydus Cadila, Strides Pharma Science Limited, Glenmark Pharmaceuticals Ltd., Wockhardt Ltd., Macleods Pharmaceuticals Ltd., Intas Pharmaceuticals Ltd., Alembic Pharmaceuticals Limited, Indoco Remedies Limited.
North America was the largest region in the grand mal seizure treatment market in 2024. The regions covered in the grand mal seizure treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the grand mal seizure treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The grand mal seizure treatment market research report is one of a series of new reports that provides grand mal seizure treatment market statistics, including grand mal seizure treatment industry global market size, regional shares, competitors with a grand mal seizure treatment market share, detailed grand mal seizure treatment market segments, market trends and opportunities, and any further data you may need to thrive in the grand mal seizure treatment industry. This grand mal seizure treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Treatment for grand mal seizures involves medical interventions and management strategies aimed at controlling or minimizing the impact of tonic-clonic seizures on an individual's life. The goal is to prevent or reduce the frequency, duration, and intensity of seizures while mitigating potential medication side effects.
Key categories of grand mal seizure treatments include barbiturates, hydantoin, phenyltriazine, iminostilbenes, benzodiazepines, aliphatic carboxylic acids, and other medication classes. Barbiturates function as central nervous system depressants, serving as sedatives, hypnotics, and anticonvulsants. Diagnosis methods may encompass magnetic resonance imaging (MRI), electroencephalogram (EEG), blood tests, computed tomography (CT), and other diagnostic procedures. Treatment options include antiepileptic drugs, surgical interventions, vagus nerve stimulation, ketogenic diet, among others. These treatments cater to various end-users, including academic and research centers, neurological facilities, hospitals, and other healthcare providers.
The grand mal seizure treatment market consists of revenues earned by entities by providing deep brain stimulation, complementary therapies, and surgical procedures. The market value includes the value of related goods sold by the service provider or included within the service offering. The grand mal seizure treatment market also includes sales of brivaracetam, carbamazepine, clobazam, and felbamate. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Grand Mal Seizure Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on grand mal seizure treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for grand mal seizure treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The grand mal seizure treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Barbiturates; Hydantoin; Phenyltriazine; Iminostilbenes; Benzodiazepines; Aliphatic Carboxylic Acids; Other Types2) By Diagnosis: Magnetic Resonance Imaging (MRI); Electroencephalogram (EEG); Blood Tests; Computed Tomography (CT); Other Diagnosis
3) By Treatment: Antiepileptic Drugs; Surgery; Vagus Nerve Stimulation; Ketogenic Diet; Other Treatments
4) By End Users: Academic and Research Centers; Neurological Centers; Hospitals; Other End Users
Subsegments:
1) By Barbiturates: Phenobarbital; Primidone2) By Hydantoins: Phenytoin
3) By Phenyltriazines: Lamotrigine
4) By Iminostilbenes: Carbamazepine; Oxcabazepine
5) By Benzodiazepines: Clonazepam; Diazepam; Lorazepam
6) By Aliphatic Carboxylic Acids: Valproic Acid; Divalproex Sodium
7) By Other Types: Levetiracetam; Topiramate; Zonisamide
Companies Mentioned: Novartis AG; Sanofi S.A.; GlaxoSmithKline PLC; Takeda Pharmaceuticals Inc.; Mylan N.V.; UCB Celltech Ltd.; Sun Pharmaceutical Industries Ltd.; Teva Pharmaceutical Industries Ltd.; Aurobindo Pharma Limited; Lupin Pharmaceuticals Inc.; Dr. Reddy's Laboratories Ltd.; Cipla Inc.; Apotex Inc.; Hikma Pharmaceuticals PLC; Alkem Laboratories Ltd.; Jubilant Life Sciences Limited; Torrent Pharmaceuticals Ltd.; Zydus Cadila; Strides Pharma Science Limited; Glenmark Pharmaceuticals Ltd.; Wockhardt Ltd.; Macleods Pharmaceuticals Ltd.; Intas Pharmaceuticals Ltd.; Alembic Pharmaceuticals Limited; Indoco Remedies Limited
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Grand Mal Seizure Treatment market report include:- Novartis AG
- Sanofi S.A.
- GlaxoSmithKline PLC
- Takeda Pharmaceuticals Inc.
- Mylan N.V.
- UCB Celltech Ltd.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Aurobindo Pharma Limited
- Lupin Pharmaceuticals Inc.
- Dr. Reddy's Laboratories Ltd.
- Cipla Inc.
- Apotex Inc.
- Hikma Pharmaceuticals PLC
- Alkem Laboratories Ltd.
- Jubilant Life Sciences Limited
- Torrent Pharmaceuticals Ltd.
- Zydus Cadila
- Strides Pharma Science Limited
- Glenmark Pharmaceuticals Ltd.
- Wockhardt Ltd.
- Macleods Pharmaceuticals Ltd.
- Intas Pharmaceuticals Ltd.
- Alembic Pharmaceuticals Limited
- Indoco Remedies Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.08 Billion |
| Forecasted Market Value ( USD | $ 2.58 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |