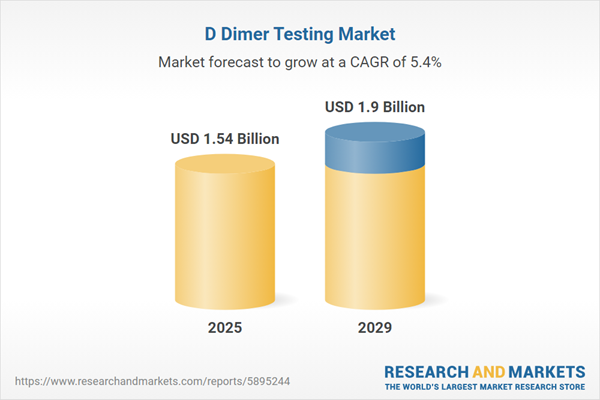
The D dimer testing market size is expected to see strong growth in the next few years. It will grow to $1.9 billion in 2029 at a compound annual growth rate (CAGR) of 5.4%. The growth in the forecast period can be attributed to the increasing geriatric population, growth in point-of-care testing (POCT), integration with risk assessment algorithms, increased awareness and education campaigns, regulatory guidance and standardization. Major trends in the forecast period include biomarker combinations for enhanced diagnosis, focus on rapid and accurate results, d-dimer testing in COVID-19 management, remote monitoring and home-based testing kits, continuous monitoring, and serial testing.
The anticipated increase in the prevalence of cancer is poised to be a significant driver for the growth of the d-dimer testing market. Cancer, characterized by uncontrolled proliferation of abnormal cells that can invade neighboring tissues or spread to other organs, necessitates effective diagnostic and monitoring tools. D-dimer testing plays a crucial role in the management of cancer patients, aiding in diagnosis, monitoring, and treatment, as well as assessing the risk of blood clot formation - a common complication in cancer. As of January 2022, research from the American Cancer Society indicates a substantial burden of cancer, with an anticipated 609,360 cancer deaths and 1.9 million new cancer cases in the US in 2022. The increasing prevalence of cancer, with about 1,670 fatalities each day, underscores the driving force behind the growth of the d-dimer testing market.
The increasing number of surgical procedures is anticipated to drive the growth of the D-dimer testing market in the future. A surgical procedure refers to a medical intervention that employs manual or instrumental techniques to address a disease, injury, or condition. The growing prevalence of surgical procedures boosts the demand for D-dimer testing services, as these surgeries carry a risk of thrombosis, making D-dimer testing essential for postoperative monitoring to identify complications early and enhance patient outcomes. For example, in September 2023, the International Society of Aesthetic Plastic Surgery (ISAPS), a U.S.-based organization, reported that liposuction remained the most common surgical procedure in 2022, maintaining its ranking from 2021, with over 2.3 million procedures performed, marking a 21.1% increase. Thus, the rising demand for surgical procedures is propelling the growth of the D-dimer testing market.
Major players in the d-dimer testing market are actively pursuing a strategic partnership approach to secure exclusive rights to d-dimer testing solutions, gaining a competitive advantage in the market. Strategic partnerships involve companies leveraging each other's strengths and resources for mutual benefits and success. An illustrative example is the partnership between SphingoTec GmbH, a Germany-based biotechnology company, and Rivaara Labs Pvt Ltd., an India-based medical laboratory and healthcare company. Established in March 2022, this partnership grants Rivaara exclusive rights to market and distribute SphingoTec's point-of-care tests featuring innovative biomarkers in the Indian subcontinent. These biomarkers include proenkephalin (penKid) for kidney function assessment, bioactive adrenomedullin (bio-ADM) for endothelial function evaluation, and dipeptidyl peptidase 3 (DPP3) as a biomarker for cardiac depression. These pioneering markers, along with commonly used parameters such as procalcitonin, troponin, D-dimer, NT-proBNP, and TSH, enhance diagnostic capabilities.
Leading companies in the D-dimer testing market are creating innovative products, such as D-dimer test kits, to enhance the accuracy and speed of diagnosing blood clot-related conditions. A D-dimer test kit is a medical diagnostic tool used to measure the levels of D-dimer, a fibrin degradation product, in the blood, aiding in the assessment of blood clots and conditions like deep vein thrombosis, pulmonary embolism, or disseminated intravascular coagulation. For example, in July 2024, Bionote Inc., a South Korea-based manufacturer of medical equipment, launched the Vcheck D-dimer 5 Tests/Kit. This new D-dimer 5-Test Kit, designed specifically for Vcheck immunodiagnostic equipment, is tailored for veterinary clinics. It assists in diagnosing conditions such as thrombosis and disseminated intravascular coagulation (DIC), making it particularly suitable for smaller clinics with fewer emergency cases.
In March 2023, Werfen S.A., a leading diagnostic company based in Spain, successfully acquired Immucor Inc. for a significant sum of $2 billion. This strategic acquisition was pursued by Werfen with the objective of broadening its range of products and reinforcing its standing within the global market for transfusion and transplantation diagnostics. The acquisition of Immucor Inc., a distinguished US-based manufacturer known for producing d-dimer test kits, encompassing enzyme-linked immunosorbent assay (ELISA) technology, as well as transfusion and transplant diagnostic products, is expected to provide Werfen with access to Immucor's extensive global presence and resources.
Major companies operating in the D dimer testing market include Kaiser Foundation Health Plan Inc., F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Abbott Laboratories, Siemens Healthcare GmbH, Becton Dickinson And Company (BD), Laboratory Corporation, Quest Diagnostics Inc., bioMérieux SA, Beckman Coulter Inc., Quidel Corporation, QuidelOrtho Corporation, Sysmex Corporation, Horiba Ltd., Werfen SA, Helena Laboratories Corporation, Abcam plc, LumiraDx Limited, Personalabs, Sekisui Diagnostics LLC, Diagnostica Stago S.A.S., RayBiotech Inc., CTK Biotech Inc., Diazyme Laboratories Inc., AdvaCare Pharma USA, UCP Biosciences Inc., AccuBioTech Co Ltd., Response Biomedical Corporation.
North America was the largest region in the D dimer testing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the d dimer testing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the d dimer testing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
D-dimer testing involves a laboratory blood test designed to measure the levels of d-dimer, a protein fragment formed when blood clots break down in the body. This test serves as a diagnostic tool to either confirm or rule out the presence of blood clots, particularly in conditions such as deep vein thrombosis (DVT) or pulmonary embolism (PE).
The primary categories of d-dimer testing encompass clinical laboratory tests and point-of-care tests. Clinical laboratory tests encompass a broad range of diagnostic procedures conducted on patient samples within laboratory settings. These tests involve various products such as analyzers, reagents, and consumables, employing methods such as enzyme-linked immunosorbent assay (ELISA), latex-enhanced immuno-turbidimetric assays, and others. They serve multiple applications including detecting conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), disseminated intravascular coagulation (DIC), among others. These tests are utilized by various end-users including hospitals, academic and research institutes, diagnostic centers, and other medical facilities.
The d-dimer testing market research report is one of a series of new reports that provides d-dimer testing market statistics, including d-dimer testing industry global market size, regional shares, competitors with a d-dimer testing market share, detailed d-dimer testing market segments, market trends, and opportunities, and any further data you may need to thrive in the d-dimer testing industry. This d-dimer testing market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The d-dimer testing market consists of revenues earned by entities by providing services such as sample collection, sample processing, exclusion of blood clots, and risk assessment and stratification. The market value includes the value of related goods sold by the service provider or included within the service offering. The d-dimer testing market also includes sales of d-dimer assay kits, d-dimer rapid tests, automated d-dimer analyzers, and point-of-care test kits that are used in providing d-dimer testing services. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
D Dimer Testing Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on d dimer testing market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for d dimer testing? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The d dimer testing market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Test: Clinical Laboratory Tests; Point-of-Care Tests2) By Product: Analyzers; Reagents and Consumables
3) By Method: Enzyme-linked Immunosorbent Assay (ELISA); Latex-enhanced Immuno-turbidimetric Assays; Fluorescence Immunoassays; Other Testing Method
4) By Application: Deep Vein Thrombosis (DVT); Pulmonary Embolism (PE); Disseminated Intravascular Coagulation (DIC); Other Application
5) By End-User: Hospitals; Academic and Research Institutes; Diagnostic Centers; Other End-User
Subsegments:
1) By Clinical Laboratory Tests: Enzyme-Linked Immunosorbent Assay (Elisa); Chemiluminescent Immunoassay (CLIA); Latex Agglutination Assays; Other Laboratory Testing Methods2) By Point-of-Care Tests: Rapid Test Kits; Portable Testing Devices; Lateral Flow Assays; Other Point-of-Care Testing Methods
Key Companies Mentioned: Kaiser Foundation Health Plan Inc.; F. Hoffmann-La Roche Ltd.; Thermo Fisher Scientific Inc.; Abbott Laboratories; Siemens Healthcare GmbH
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Kaiser Foundation Health Plan Inc.
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Siemens Healthcare GmbH
- Becton Dickinson And Company (BD)
- Laboratory Corporation
- Quest Diagnostics Inc.
- bioMérieux SA
- Beckman Coulter Inc.
- Quidel Corporation
- QuidelOrtho Corporation
- Sysmex Corporation
- Horiba Ltd.
- Werfen SA
- Helena Laboratories Corporation
- Abcam plc
- LumiraDx Limited
- Personalabs
- Sekisui Diagnostics LLC
- Diagnostica Stago S.A.S.
- RayBiotech Inc.
- CTK Biotech Inc.
- Diazyme Laboratories Inc.
- AdvaCare Pharma USA
- UCP Biosciences Inc.
- AccuBioTech Co Ltd.
- Response Biomedical Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.54 Billion |
| Forecasted Market Value ( USD | $ 1.9 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |