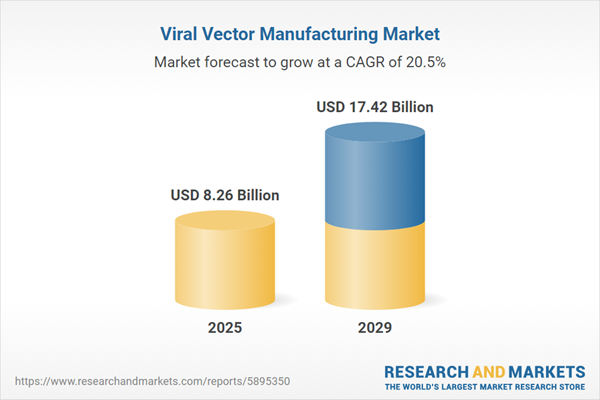
The viral vector manufacturing market size is expected to see exponential growth in the next few years. It will grow to $17.42 billion in 2029 at a compound annual growth rate (CAGR) of 20.5%. The growth in the forecast period can be attributed to expanding applications in oncology, global collaborations in gene therapy research, adoption of viral vectors in vaccine development, continued clinical trials and commercialization. Major trends in the forecast period include technological innovations in vector production, emergence of next-generation vectors, regulatory compliance and standardization, collaborations and partnerships, and global expansion of manufacturing facilities.
The forecast of 20.5% growth over the next five years reflects a slight reduction of 0.2% from the previous projection. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff barriers could challenge U.S. gene therapy developers by escalating prices of HEK293 cell lines and transfection reagents sourced from the UK and Switzerland, resulting in delayed viral vector production and increasing cell and gene therapy research and development expenses. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The rising prevalence of cancer and infectious diseases is expected to drive growth in the viral vector manufacturing market. An infectious disease is caused by a virus or its toxic byproducts, spreading to susceptible hosts through contact with infected individuals, animals, or objects. Cancer encompasses a wide range of illnesses that can develop in any organ or tissue when abnormal cells grow uncontrollably, invade neighboring tissues, and spread to other parts of the body. Viral vectors produce tumor antigens, or proteins on tumor cells, to trigger the body's anticancer immune response. These vectors have also been utilized as vaccines in pre-clinical and clinical trials for various infectious diseases. For instance, in July 2024, the Australian Institute of Health and Welfare reported that the number of diagnosed cancer cases in Australia increased from 160,570 in 2022 to 164,694 in 2023, indicating a significant rise. Additionally, in August 2024, the UK Health Security Agency reported 368 measles cases in England for 2023, nearly a sevenfold increase from the 53 cases recorded in 2022, with the West Midlands and London accounting for 44% and 33% of the cases, respectively. Thus, the increasing prevalence of cancer and infectious diseases is driving the growth of the viral vector manufacturing market.
The growth of the viral vector manufacturing market is further propelled by the expanding pipeline of gene therapy. Gene therapy involves manipulating or replacing genetic material within an individual's cells to treat or prevent diseases. Viral vector manufacturing plays a crucial role in this domain by providing efficient and scalable platforms for producing viral vectors, which serve as vital vehicles for delivering therapeutic genes into target cells. This advancement facilitates the development of innovative gene therapies. In January 2023, the American Society of Gene & Cell Therapy reported a 7% growth in the US gene, cell, and RNA therapy pipeline in 2022, totaling 3,726 therapies. The preclinical stage dominates with 70% of therapies, and oncology leads as the most active therapeutic area, hosting over 1,300 gene and cell therapy candidates. The expanding pipeline of gene therapy is a driving force behind the growth of the viral vector manufacturing market.
Product innovation is a prominent trend in the viral vector manufacturing market, with major companies introducing advanced solutions to maintain and strengthen their positions. In August 2022, Merck KGaA, a German-based science and technology company, unveiled the VirusExpress 293 Adeno-Associated Virus (AAV) Production Platform. As one of the first Contract Development and Manufacturing Organizations (CDMOs) and technology creators to provide a complete viral vector manufacturing package, including AAV, Lentiviral, CDMO, CTO, and process development, Merck KGaA addresses the need for a comprehensive solution in the industry. The VirusExpress 293 AAV Production Platform allows biopharmaceutical companies to streamline process development, reduce time and costs, and accelerate the timeline for clinical manufacture.
Major players in the viral vector manufacturing market are emphasizing the development of advanced products, particularly viral vector platforms tailored for efficient viral vector production and manufacturing. Viral vector platforms are comprehensive systems designed to streamline the production and processing of viral vectors for various applications, including gene therapy and vaccine development. In May 2023, AGC Biologics, a US-based global biopharmaceutical Contract Development and Manufacturing Organization (CDMO), launched the BravoAAV and ProntoLVV platforms. These platforms are specifically designed to provide flexible and accelerated vector development and manufacturing for cell and gene therapy programs. Offering GMP product delivery in nine months, supported by a global regulatory and supply network, and in-house plasmid DNA services, these platforms play a crucial role in meeting the increasing demand for efficient development and delivery of life-changing gene therapies and vaccines. Custom processes, templated material approaches, and prequalified analytical methods further contribute to the significance of these platforms in the viral vector manufacturing market, which is projected to reach $5.5 billion by 2035.
In January 2024, Oxford Biomedica Plc, a UK-based biotechnology firm, acquired ABL Europe from Institut Mérieux for $16 million (15 million euros). This acquisition enables Oxford Biomedica to utilize the production facilities in Lyon and Strasbourg, France, enhancing its multi-viral vector CDMO capabilities with a total of six manufacturing sites across the EU, U.S., and U.K. ABL Europe is a France-based contract development and manufacturing organization (CDMO) specializing in viral vector manufacturing services.
Major companies operating in the viral vector manufacturing market include Sanofi S.A., Thermo Fisher Scientific Inc., Merck Group, FUJIFILM Holdings Corporation, Lonza Group, Catalent Inc., Sartorius AG, Charles River Laboratories International Inc., AGC Biologics, Ultragenyx Pharmaceutical Inc., Novasep Holding SAS, Aldevron LLC, Oxford Biomedica plc, LakePharma Inc., Voyager Therapeutics Inc., Mustang Bio Inc., Regenxbio Inc., VGXI Inc., BioNTech IMFS GmbH, FinVector Oy, Vigene Biosciences Inc., Univercells Technologies, Sirion-Biotech GmbH, Cevec Pharmaceuticals GmbH, Batavia Biosciences BV.
North America was the largest region in the viral vector manufacturing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the viral vector manufacturing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the viral vector manufacturing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The viral vector manufacturing market research report is one of a series of new reports that provides viral vector manufacturing market statistics, including viral vector manufacturing industry global market size, regional shares, competitors with a viral vector manufacturing market share, detailed viral vector manufacturing market segments, market trends and opportunities, and any further data you may need to thrive in the viral vector manufacturing industry. This viral vector manufacturing market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Viral vector manufacturing involves the creation of viral vectors, tools designed to transport genetic material into cells. These vectors serve as carriers for gene transfer, enabling specific cell types or tissues to express therapeutic genes.
The primary categories of viral vector manufacturing include adenoviral vectors, adeno-associated viral vectors, lentiviral vectors, retroviral vectors, and other variations. Adenoviral vectors, for instance, are double-stranded DNA vectors lacking an outer casing. These vectors find application across a spectrum of diseases including cancer, genetic disorders, infectious diseases, and more, utilizing workflows involving both upstream and downstream processing. These technologies have diverse applications in gene and cell therapy development, vaccine research, and the discovery phases of biopharmaceuticals and pharmaceuticals. They are extensively utilized in research institutions, biotechnology and pharmaceutical companies, and various other related fields for biomedical research purposes.
The viral vector manufacturing market includes revenues earned by entities through the production, packaging, and labeling of viral vector vaccines. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Viral Vector Manufacturing Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on viral vector manufacturing market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for viral vector manufacturing? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The viral vector manufacturing market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Adenoviral Vectors; Adeno-Associated Viral Vectors; Lentiviral Vectors; Retroviral Vectors; Other Types2) By Disease: Cancer; Genetic Disorders; Infectious Diseases; Other Diseases
3) By Workflow: Upstream Processing; Downstream Processing
4) By Application: Gene and Cell Therapy Development; Vaccine Development; Biopharmaceutical and Pharmaceutical Discovery; Biomedical Research
5) By End-User: Research Organizations; Biotech and Pharmaceutical Companies; Others End Users
Subsegments:
1) By Adenoviral Vectors: Serotype 5; Serotype 2; Serotype 262) By Adeno-Associated Viral Vectors: AAV2; AAV5; AAV8; AAV9; Others
3) By Lentiviral Vectors: HIV-1-Based Lentiviral Vectors; SIV-Based Lentiviral Vectors; Others
4) By Retroviral Vectors: Moloney Murine Leukemia Virus (MoMLV); Gammaretroviral Vectors; Others
5) By Other Types: Sendai Viral Vectors; Vesicular Stomatitis Virus (VSV)-Based Vectors; Measles Virus Vectors; Others
Companies Mentioned: Sanofi S.A.; Thermo Fisher Scientific Inc.; Merck Group; FUJIFILM Holdings Corporation; Lonza Group; Catalent Inc.; Sartorius AG; Charles River Laboratories International Inc.; AGC Biologics; Ultragenyx Pharmaceutical Inc.; Novasep Holding SAS; Aldevron LLC; Oxford Biomedica plc; LakePharma Inc.; Voyager Therapeutics Inc.; Mustang Bio Inc.; Regenxbio Inc.; VGXI Inc.; BioNTech IMFS GmbH; FinVector Oy; Vigene Biosciences Inc.; Univercells Technologies; Sirion-Biotech GmbH; Cevec Pharmaceuticals GmbH; Batavia Biosciences BV.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Viral Vector Manufacturing market report include:- Sanofi S.A.
- Thermo Fisher Scientific Inc.
- Merck Group
- FUJIFILM Holdings Corporation
- Lonza Group
- Catalent Inc.
- Sartorius AG
- Charles River Laboratories International Inc.
- AGC Biologics
- Ultragenyx Pharmaceutical Inc.
- Novasep Holding SAS
- Aldevron LLC
- Oxford Biomedica plc
- LakePharma Inc.
- Voyager Therapeutics Inc.
- Mustang Bio Inc.
- Regenxbio Inc.
- VGXI Inc.
- BioNTech IMFS GmbH
- FinVector Oy
- Vigene Biosciences Inc.
- Univercells Technologies
- Sirion-Biotech GmbH
- Cevec Pharmaceuticals GmbH
- Batavia Biosciences BV.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 8.26 Billion |
| Forecasted Market Value ( USD | $ 17.42 Billion |
| Compound Annual Growth Rate | 20.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |