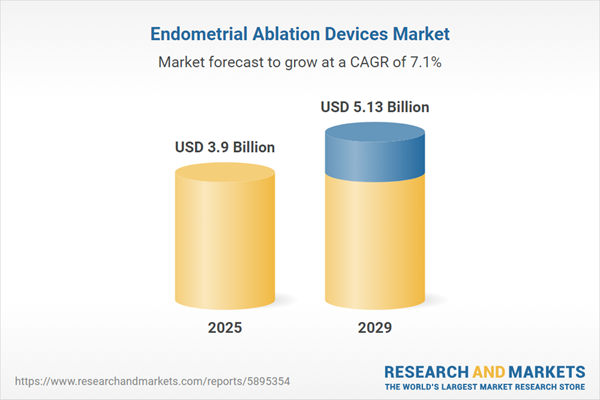
The endometrial ablation devices market size is expected to see strong growth in the next few years. It will grow to $5.13 billion in 2029 at a compound annual growth rate (CAGR) of 7.1%. The growth in the forecast period can be attributed to growing awareness and education, emphasis on fertility preservation, personalized medicine approach, regulatory support and guidelines, and focus on office-based procedures. Major trends in the forecast period include the integration of telemedicine and remote consultations, standardization of protocols, the shift towards non-hormonal treatment, integration of patient-centric care, and development of safer procedures.
The growth of the endometrial ablation devices market is anticipated to be fueled by the increasing prevalence of gynecological disorders. Gynecological disorders encompass various medical conditions affecting the female reproductive system, including menstrual disorders, endometriosis, polycystic ovary syndrome (PCOS), uterine fibroids, pelvic inflammatory disease (PID), and ovarian cysts. Endometrial ablation devices play a crucial role in treating these disorders by destroying the endometrial lining, providing relief from heavy menstrual bleeding, and addressing conditions such as adenomyosis and endometrial hyperplasia. As of June 2023, the World Health Organization reports that PCOS affects 8-13% of reproductive-aged women, with up to 70% of affected women globally going misidentified. This increasing prevalence underscores the driving force behind the growth of the endometrial ablation devices market.
The growth in healthcare spending is anticipated to drive the expansion of the endometrial ablation devices market in the coming years. Healthcare expenditure refers to the total financial resources allocated for healthcare services, products, and infrastructure within a specific country, region, or organization. Targeted and efficient healthcare spending enhances the connectivity, safety, and overall effectiveness of endometrial ablation procedures, leading to improved outcomes for women with gynecological conditions. For instance, in May 2023, the Office for National Statistics, a UK-based government department, reported that healthcare spending in the UK reached approximately $354.88 billion (£283 billion), reflecting a nominal increase of 0.7% compared to the previous year. Therefore, the rise in healthcare spending is fueling the growth of the endometrial ablation devices market.
Leading companies in the endometrial ablation devices market are concentrating on securing regulatory approvals to introduce innovative products and expand their market presence. Regulatory approvals involve formal authorization granted by government agencies or regulatory bodies, ensuring that a medical device, drug, or treatment meets the required safety and efficacy standards before it can be sold or utilized in a specific region. For example, in February 2023, Hologic Inc., a US-based health technology firm, received regulatory approval for its NovaSure V5 Global Endometrial Ablation Device in both Canada and Europe. This device, designed for the treatment of abnormal uterine bleeding through a minimally invasive procedure, includes advanced technologies such as SmartDepth for precise ablation and SureClear for effective suction during the procedure. It allows for personalized treatment for individual patients, with clinical outcomes showing a 97% satisfaction rate, 75% of patients experiencing amenorrhea after five years, and 86% avoiding hysterectomy after ten years.
Key players in the endometrial ablation devices market are also working on developing advanced solutions like field ablation systems to improve efficacy and patient comfort. A field ablation system is a medical device used to treat abnormal tissue, particularly in the heart, by applying energy (such as radiofrequency, laser, or cryotherapy) to a specific area. For instance, in January 2024, Abbott Laboratories, a US-based medical device company, launched its Volt Pulsed Field Ablation (PFA) System, marking the beginning of human trials aimed at treating atrial fibrillation (AFib). This cutting-edge technology uses pulsed field energy to selectively target heart muscle cells, minimizing collateral damage to surrounding tissues compared to traditional thermal ablation techniques. The system features a balloon-in-basket catheter design for greater precision and stability, and integrates with the EnSite X cardiac mapping platform to improve procedural accuracy. Initial trials are being conducted in Australia, with plans for global expansion, positioning Abbott as a leader in AFib treatment as demand for advanced therapies grows.
In August 2022, Medtronic, an Ireland-based medical device company, acquired Affera for $925 million. This acquisition allows Medtronic to enhance its cardiac ablation portfolio and advance electrophysiology technology, improving treatment options for patients with heart rhythm disorders. This strategic move strengthens Medtronic's ability to support physicians and increases its market presence in the growing cardiac care sector. Affera, a US-based medical technology company, focuses on developing cardiac ablation devices for treating arrhythmias.
Major companies operating in the endometrial ablation devices market include Johnson and Johnson Private Limited, Medtronic Plc., Stryker Corporation, Boston Scientific Corporation, Olympus Corporation, Smith And Nephew plc., Hologic Inc., C R.Bard Inc., Cooper Companies Inc., Cook Medical, Karl Storz GmbH And Co. KG, Biotronik SE And Co. KG, B. Braun Medical Inc., Richard Wolf GmbH, Aesculap Inc., AngioDynamics, Gyrus ACMI Inc., Minerva Surgical Inc., Lina Medical ApS, Biolitec AG, AEGEA Medical Inc., Albyn Medical,Channel Medsystems Inc., CathRX Ltd, Ecleris Srl., Idoman Teoranta, Surkon Medical Co. Ltd, Omnitech Systems Inc., Veldana Medical SA.
North America was the largest region in the endometrial ablation devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the endometrial ablation devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the endometrial ablation devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Endometrial ablation devices encompass a range of medical tools specifically engineered for conducting procedures involving the removal or destruction of the uterine lining. These devices are instrumental in addressing gynecological conditions associated with irregular uterine bleeding.
The various categories of endometrial ablation devices exist, including hysteroscopy devices, thermal balloon ablators, radiofrequency endometrial ablation devices, hydrothermal ablators, electrical ablators, and others. Hysteroscopy devices encompass a suite of medical tools employed in the diagnostic and therapeutic practice of hysteroscopy. These technologies, comprising radiofrequency ablation, cryoablation, hydrothermal ablation, thermal balloon procedures, and hysteroscopic ablation, cater to diverse end-users such as ambulatory surgery centers, clinics, and hospitals.
The endometrial ablation devices market research report is one of a series of new reports that provides endometrial ablation devices market statistics, including endometrial ablation devices industry global market size, regional shares, competitors with an endometrial ablation devices market share, detailed endometrial ablation devices market segments, market trends and opportunities, and any further data you may need to thrive in the endometrial ablation devices industry. This endometrial ablation devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The endometrial ablation devices market consists of sales of radiofrequency ablation devices, cryoablation devices, hydrothermal devices, and microwave ablation devices. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Endometrial Ablation Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on endometrial ablation devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for endometrial ablation devices? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The endometrial ablation devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Device Type: Hysteroscopy Devices; Thermal Balloon Ablators; Radiofrequency Endometrial Ablation Devices; Hydrothermal Ablators; Electrical Ablators; Other Device Types2) By Technology: Radiofrequency Ablation; Cryoablation; Hydrothermal Ablation; Thermal Balloon; Hysteroscopic Ablation; Other Technologies
3) By End User: Ambulatory Surgery Center; Clinic; Hospital
Subsegments:
1) By Hysteroscopy Devices: Hysteroscopic Resectoscopes; Operative Hysteroscopes2) By Thermal Balloon Ablators: Single-Use Balloon Catheters; Multi-Use Balloon Systems
3) By Radiofrequency Endometrial Ablation Devices: Bipolar Rf Systems; Monopolar Rf Systems
4) By Hydrothermal Ablators: Heated Saline Infusion Systems; Water-Based Ablation Devices
5) By Electrical Ablators: Electrosurgical Systems; Electrical Current-Based Devices
6) By Other Device Types: Cryoablation Devices; Microwave Endometrial Ablation Systems; Laser Ablation Devices
Key Companies Mentioned: Johnson and Johnson Private Limited; Medtronic Plc.; Stryker Corporation; Boston Scientific Corporation; Olympus Corporation
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Johnson and Johnson Private Limited
- Medtronic Plc.
- Stryker Corporation
- Boston Scientific Corporation
- Olympus Corporation
- Smith And Nephew plc.
- Hologic Inc.
- C R.Bard Inc.
- Cooper Companies Inc.
- Cook Medical
- Karl Storz GmbH And Co. KG
- Biotronik SE And Co. KG
- B. Braun Medical Inc.
- Richard Wolf GmbH
- Aesculap Inc.
- AngioDynamics
- Gyrus ACMI Inc.
- Minerva Surgical Inc.
- Lina Medical ApS
- Biolitec AG
- AEGEA Medical Inc.
- Albyn Medical
- Channel Medsystems Inc.
- CathRX Ltd
- Ecleris Srl.
- Idoman Teoranta
- Surkon Medical Co. Ltd
- Omnitech Systems Inc.
- Veldana Medical SA.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.9 Billion |
| Forecasted Market Value ( USD | $ 5.13 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |