Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
A notable shift is occurring toward more targeted and individualized therapies, supported by ongoing innovations such as calcitonin gene-related peptide (CGRP) inhibitors and gepants, which are demonstrating promising clinical outcomes. Increased recognition of migraine's public health and economic impacts has also driven investment in R&D by both public and private sectors. With pharmaceutical companies focusing on introducing next-generation drugs and biologics, the market continues to evolve toward more effective and personalized treatment strategies. These innovations, along with greater awareness and improved access to healthcare, are expected to support sustained market growth.
Key Market Drivers
High Prevalence of Migraine
The widespread occurrence of migraine is a key driver fueling the growth of the Migraine Therapeutics Market. Migraine affects individuals of all ages, genders, and demographics, making it a pervasive public health issue. The condition is a leading cause of disability and contributes to significant losses in productivity, impacting both individuals and economies.As per a March 2024 WHO report, around 40% of the global population - approximately 3.1 billion people - suffered from migraines and other headache disorders as of 2021. The condition shows a higher prevalence among women, likely due to hormonal factors. Migraine ranks among the top three neurological disorders from childhood through late adulthood. This widespread burden has led to increased attention from healthcare stakeholders, driving awareness, diagnosis, and treatment. The rising recognition of migraine's impact on quality of life has also encouraged the development and uptake of more effective therapies, including CGRP inhibitors and gepants, further expanding the market.
Key Market Challenges
Drug Development Complexity
Developing effective migraine therapeutics presents significant challenges due to the complex nature of the condition. Migraine manifests with diverse symptoms and triggers, requiring a deep understanding of its neurological basis for effective drug design. Identifying precise molecular targets is difficult, as the underlying mechanisms of migraine are still not fully understood.The clinical trial process for migraine treatments can be lengthy and costly, involving large patient populations and extended follow-up periods. Subjective symptom reporting and placebo responses add further complexity. Regulatory agencies such as the FDA require comprehensive evidence of safety and efficacy, necessitating rigorous testing and data collection. In addition, evolving clinical trial endpoints demand constant adaptation from researchers and sponsors. These challenges, combined with high development costs, can delay innovation and restrict market entry, particularly for smaller firms.
Key Market Trends
Precision Medicine
Precision medicine is emerging as a transformative trend in the Migraine Therapeutics Market, offering more effective and individualized treatment strategies. By utilizing genetic, molecular, and clinical data, precision medicine allows for the customization of therapies based on a patient’s unique migraine profile. Advances in genetic testing and biomarker analysis enable pharmaceutical developers and healthcare providers to better identify subgroups of patients who are most likely to benefit from specific treatments. This targeted approach improves treatment outcomes, reduces side effects, and enhances patient satisfaction. For pharmaceutical companies, precision medicine increases the efficiency of drug development and can lead to faster regulatory approvals. It also offers the potential for higher market differentiation and pricing power. As demand grows for more personalized healthcare solutions, the integration of precision medicine into migraine treatment protocols is expected to expand significantly, reshaping the market landscape and improving therapeutic success rates.Key Players Profiled in this Migraine Therapeutics Market Report
- Amgen Inc.
- Teva Pharmaceutical Industries Limited
- GlaxoSmithKline plc
- Eli Lilly and Company
- Bausch Health Companies Inc.
- Novartis AG
- AbbVie Inc.
- Lundbeck Inc
- Dr. Reddy's Laboratories Limited
- Impel Pharmaceuticals Inc
Report Scope:
In this report, the Migraine Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Migraine Therapeutics Market, Therapeutics:
- Pain-relieving Medications (Analgesics, Triptans, Ergot Alkaloids, Others)
- Preventive Medications (Blood pressure-lowering Medications, Anticonvulsant Drugs, Calcitonin Gene-related Peptide (CGRP) Antagonists, Other Preventative Therapies)
Migraine Therapeutics Market, Route of Administration:
- Oral & Nasal
- Injectables
Migraine Therapeutics Market, Distribution Channel:
- Retail Pharmacy
- Hospital Pharmacy
- Others
Migraine Therapeutics Market, by Region:
- North America
- United States
- Mexico
- Canada
- Europe
- France
- Germany
- United Kingdom
- Italy
- Spain
- Asia-Pacific
- China
- India
- South Korea
- Japan
- Australia
- South America
- Brazil
- Argentina
- Colombia
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Migraine Therapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The leading companies profiled in this Migraine Therapeutics market report include:- Amgen Inc.
- Teva Pharmaceutical Industries Limited
- GlaxoSmithKline plc
- Eli Lilly and Company
- Bausch Health Companies Inc.
- Novartis AG
- AbbVie Inc.
- Lundbeck Inc
- Dr. Reddy's Laboratories Limited
- Impel Pharmaceuticals Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | May 2025 |
| Forecast Period | 2024 - 2030 |
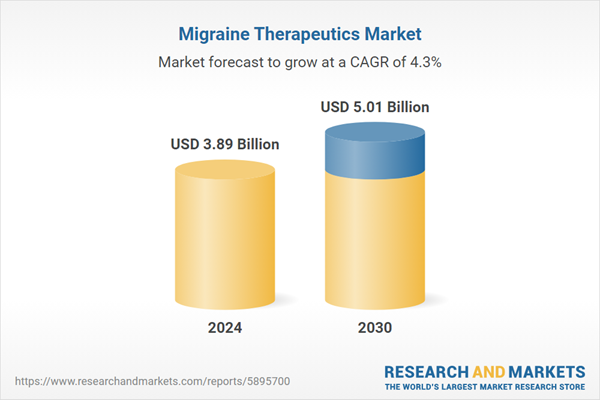
| Estimated Market Value ( USD | $ 3.89 Billion |
| Forecasted Market Value ( USD | $ 5.01 Billion |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |