Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Their immunomodulatory and anti-inflammatory properties, combined with low immunogenicity, make them suitable for applications in orthopedics, neurology, cardiology, and autoimmune diseases. Increasing research investments and favorable demographic trends, including an aging population with rising incidences of chronic conditions, are further propelling the market. The scope of MSC applications continues to broaden, encompassing emerging areas such as dermatology and oncology, while technological innovations and expanding clinical studies underline the potential for MSCs in both therapeutic and research domains.
Key Market Drivers
Rising Prevalence of Chronic Diseases
The growing burden of chronic illnesses such as diabetes, cardiovascular diseases, neurodegenerative conditions, and arthritis is significantly driving demand for mesenchymal stem cell-based therapies. As these long-term diseases impose substantial health and economic burdens globally, MSCs offer a regenerative and immunomodulatory approach that addresses underlying tissue damage and inflammation. Their application in chronic disease management is supported by increasing interest in cell-based therapies that promote healing and reduce dependency on symptomatic treatments. The World Health Organization attributes approximately 71% of global deaths to chronic diseases, underscoring the urgent need for innovative therapies like MSCs, which are becoming integral to modern medical approaches.Key Market Challenges
Regulatory Challenges
Navigating the regulatory environment presents a key challenge for the MSC market. Regulations vary significantly across regions, affecting the approval, manufacturing, and commercialization of MSC-based products. Adhering to Good Manufacturing Practices (GMP) and gaining regulatory approvals can be time-consuming and financially burdensome. The lack of globally harmonized standards also complicates market entry for developers and manufacturers. As the demand for MSC therapies rises, the development of streamlined and unified regulatory pathways will be critical to facilitating wider access and accelerating growth in the global market.Key Market Trends
Technological Advancements
Technological innovations are transforming the MSC landscape, enabling more efficient and scalable production, enhanced therapeutic efficacy, and targeted delivery. Advances in bioreactor technology, 3D scaffolds, and microfluidic systems have improved the reproducibility and yield of MSC cultures. Tools like CRISPR-Cas9 are being used to genetically modify MSCs to enhance regenerative or immunological capabilities. Progress in biomaterials and drug delivery platforms allows for precise MSC deployment, improving clinical outcomes. Automation and robotic systems are streamlining MSC manufacturing, while imaging techniques like MRI and bioluminescent imaging provide real-time tracking of therapeutic cells. These advancements collectively support the expansion of MSC-based applications and ensure consistency in product quality, aiding in broader adoption across healthcare systems.Key Market Players
- Thermo Fisher Scientific, Inc
- Cell Applications, Inc
- Axol Biosciences Ltd
- Cytori Therapeutics Inc
- STEMCELL Technologies
- Cyagen Biosciences
- Celprogen Inc
- BrainStorm Cell Limited
- Stemedica Cell Technologies Inc
- Merck KGaA (MilliporeSigma)
- PromoCell GmbH
Report Scope:
In this report, the Global Mesenchymal Stem Cells Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Mesenchymal Stem Cells Market, By Product & Services:
- Product
- Services
Mesenchymal Stem Cells Market, By Workflow:
- Cell Sourcing & Isolation
- Culture & Cryopreservation
- Differentiation
- Characterization
Mesenchymal Stem Cells Market, By Type:
- Autologous
- Allogeneic
Mesenchymal Stem Cells Market, By Source of Isolation:
- Bone Marrow
- Cord Blood
- Peripheral Blood
- Fallopian Tube
- Fetal Liver
- Lung
- Adipose Tissues
Mesenchymal Stem Cells Market, By Indication:
- Bone And Cartilage Repair
- Cardiovascular Disease
- Inflammatory And Immunological Diseases
- Liver Diseases
- Cancer
- GvHD
- Others
Mesenchymal Stem Cells Market, By Application:
- Disease Modelling
- Drug Development & Discovery
- Stem Cell Banking
- Tissue Engineering
- Toxicology Studies
- Others
Mesenchymal Stem Cells Market, By Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Mesenchymal Stem Cells Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific, Inc
- Cell Applications, Inc
- Axol Biosciences Ltd
- Cytori Therapeutics Inc
- STEMCELL Technologies
- Cyagen Biosciences
- Celprogen Inc
- BrainStorm Cell Limited
- Stemedica Cell Technologies Inc
- Merck KGaA (MilliporeSigma)
- PromoCell GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | May 2025 |
| Forecast Period | 2024 - 2030 |
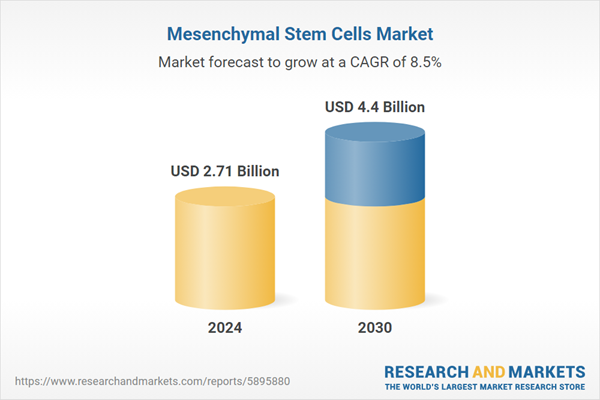
| Estimated Market Value ( USD | $ 2.71 Billion |
| Forecasted Market Value ( USD | $ 4.4 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |