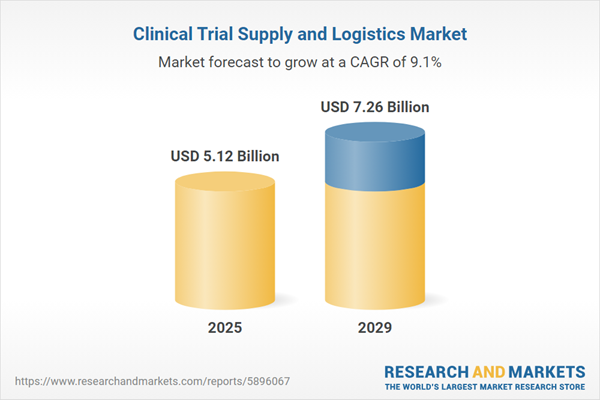
The clinical trial supply and logistics market size is expected to see strong growth in the next few years. It will grow to $7.26 billion in 2029 at a compound annual growth rate (CAGR) of 9.1%. The growth in the forecast period can be attributed to increasing demand for personalized medicine, growing number of clinical trials, rising prevalence of chronic diseases, increasing focus on patient-centric trials, increasing use of virtual trials. Major trends in the forecast period include strategic collaborations between clinical trial supply and logistics companies, pharmaceutical companies, and other stakeholders, rise in outsourcing of clinical trial services, ongoing technological advancements, increasing r&d expenditure, stringent regulatory compliance and quality assurance in clinical trial supply.
The anticipated growth in the clinical trial supply and logistics market is expected to be propelled by the increasing number of clinical trials. These trials are research studies involving human participants to assess the safety and effectiveness of new medical treatments, interventions, or diagnostic procedures. Clinical trial supply and logistics play a pivotal role in delivering essential materials, including the collection and transportation of biological specimens, investigational drugs and kits, expertise for permit applications, customs clearance, and other necessary components to ensure the smooth execution of clinical trials. For instance, as of July 2023, ClinicalTrials.gov, a registry of clinical trials by the United States National Library of Medicine, reported an increase in registered clinical studies from 399,499 in 2022 to 437,533 by July 2023, spanning all 50 states in the United States and 221 countries. Of these, 141,513 studies (31% of the total) were registered in the U.S., while 244,707 studies (53% of the total) were registered in non-U.S. locations. Therefore, the growing number of clinical trials is a driving force behind the expansion of the clinical trial supply and logistics market.
Rising R&D expenditures are anticipated to drive the growth of the clinical trial supply and logistics market in the future. R&D expenditure refers to the financial resources dedicated by organizations to research and development activities aimed at fostering innovation and creating new products or services. The clinical trial supply and logistics market significantly supports increased R&D spending by providing efficient, reliable support in the management and distribution of investigational drugs, ensuring the smooth execution of clinical trials, and facilitating faster research and development processes. For instance, the European Commission, a Belgium-based government body, reported that the EU spent €363 billion ($391.27 billion) on R&D, with an R&D expenditure of 2.27% of GDP in 2022, up from 2.25% in 2021. Consequently, the growth in R&D expenditures is fueling the expansion of the clinical trial supply and logistics market.
Leading companies in the Clinical Trial Supply and Logistics Market are concentrating on technologies like temperature-controlled healthcare shipments to enhance the integrity and reliability of products during transit, ensuring regulatory compliance and better patient outcomes. Temperature-controlled healthcare shipments refer to transporting medical products, pharmaceuticals, and biological materials that require specific temperature conditions to preserve their safety and effectiveness. This process includes specialized packaging, monitoring systems, and logistics solutions to maintain the necessary temperature range throughout transit. For instance, in February 2024, FedEx Corporation, a US-based transportation company, launched the FedEx Life Science Center. This includes FedEx Customized Freight (FCF) for handling critical pharmaceutical shipments, with features such as top boarding priority, end-to-end custodial control, and dedicated delivery options to ensure product integrity. Customers also have access to FedEx Priority Alert Plus, which provides proactive monitoring for time-sensitive shipments and offers dry ice or gel pack replenishment options.
Major players in the clinical trial supply and logistics market are placing a strategic focus on product launches, such as the introduction of electronic data capture platforms, to reshape the landscape of clinical research. An electronic data capture platform is a digital system that efficiently collects, manages, and stores clinical trial data in electronic format, enhancing the accuracy, security, and speed of the data capture process. For instance, in March 2023, Cloudbyz, a leading provider of unified clinical trial management solutions based in the U.S., unveiled EDC 2.0, a groundbreaking platform. Distinguished by features such as a user-friendly interface, advanced data security, intelligent data validation, real-time monitoring, analytics, and compliance with international regulatory standards, this product launch is highly significant in streamlining data management processes, reducing errors, and expediting regulatory approvals, ultimately enhancing efficiency in research endeavors.
In October 2022, Myonex, a clinical trial supply company based in the U.S., acquired the clinical trial supply and drug wholesale businesses from Hubertus Apotheke for an undisclosed amount. This strategic acquisition is anticipated to bolster Myonex's presence throughout the European Union in the field of clinical trial supply. Hubertus Apotheke, headquartered in Germany, is a provider of a diverse range of pharmaceuticals and medical devices.
Major companies operating in the clinical trial supply and logistics market include United Parcel Service, DHL Group, FedEx Corporation, Thermo Fisher Scientific Inc., IQVIA, ICON plc, Eurofins Scientific, Catalent Inc., Intertek Group plc, Parexel International Corporation, UDG Healthcare PLC., Almac Group, World Courier, Piramal Pharma Solutions, Sharp Clinical Services Ltd., Clinigen Group, PCI Pharma Services, Movianto Group, Cold Chain Technologies, Biocair International Limited, QuickSTAT, Wasdell Group, Klifo A/S, TrakCel, Packaging Coordinators Inc., Biotec Services International Limited, Proventa International, Inceptua Group.
North America was the largest region in the clinical trial supply and logistics market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial supply and logistics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the clinical trial supply and logistics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Clinical trial supply and logistics involve the systematic process of ensuring the timely and cost-effective delivery of necessary supplies, equipment, and medications for the execution of a clinical study. This critical procedure is designed to facilitate the safe and efficient conduct of the study while emphasizing the reduction of costs.
The primary services provided in clinical trial supply and logistics encompass logistics and distribution, storage and retention, packaging labeling and blinding, manufacturing, comparator sourcing, and others. Logistics involves the strategic planning of moving commodities, while distribution executes the conveyance of these goods through careful planning. The different clinical trial phases include phase I, phase II, phase III, and phase IV, covering various areas such as oncology, cardiovascular diseases, respiratory diseases, CNS (central nervous system) and mental disorders, among others. These services are utilized by diverse end-users, including pharmaceuticals, biologicals, and medical devices.
The clinical trial supply and logistics market research report is one of a series of new reports that provides clinical trial supply and logistics market statistics, including clinical trial supply and logistics industry global market size, regional shares, competitors with a clinical trial supply and logistics market share, detailed clinical trial supply and logistics market segments, market trends and opportunities, and any further data you may need to thrive in the clinical trial supply and logistics industry. This clinical trial supply and logistics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The clinical trial supply and logistics market includes revenues earned by entities by providing services such as clinical supply planning, import and export compliance, inventory management, packaging services, and real-time track and trace and delivery. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Clinical Trial Supply and Logistics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on clinical trial supply and logistics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for clinical trial supply and logistics? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The clinical trial supply and logistics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Service: Logistics and Distribution; Storage and Retention; Packaging Labelling and Blinding; Manufacturing; Comparator Sourcing; Other Services2) By Phase: Phase I; Phase II; Phase III; Phase IV
3) By Area: Oncology; Cardiovascular Diseases; Respiratory Diseases; CNS and Mental Disorders; Other Areas
4) By End-User: Pharmaceuticals; Biologicals; Medical Device
Subsegments:
1) By Logistics and Distribution: Transportation Management; Cold Chain Logistics; Customs Clearance; Inventory Management2) By Storage and Retention: Controlled Storage; Long-Term Storage; Short-Term Storage
3) By Packaging, Labeling, and Blinding: Primary Packaging; Secondary Packaging; Label Design and Printing; Blinding Solutions
4) By Manufacturing: Clinical Supply Manufacturing; Batch Production; Quality Control and Assurance
5) By Comparator Sourcing: Sourcing Services; Regulatory Compliance; Inventory Management For Comparators
6) By Other Services: Regulatory Affairs Consulting; Clinical Trial Management; Data Management and Analysis; Training and Support Services
Key Companies Mentioned: United Parcel Service; DHL Group; FedEx Corporation; Thermo Fisher Scientific Inc.; IQVIA
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- United Parcel Service
- DHL Group
- FedEx Corporation
- Thermo Fisher Scientific Inc.
- IQVIA
- ICON plc
- Eurofins Scientific
- Catalent Inc.
- Intertek Group plc
- Parexel International Corporation
- UDG Healthcare PLC.
- Almac Group
- World Courier
- Piramal Pharma Solutions
- Sharp Clinical Services Ltd.
- Clinigen Group
- PCI Pharma Services
- Movianto Group
- Cold Chain Technologies
- Biocair International Limited
- QuickSTAT
- Wasdell Group
- Klifo A/S
- TrakCel
- Packaging Coordinators Inc.
- Biotec Services International Limited
- Proventa International
- Inceptua Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 5.12 Billion |
| Forecasted Market Value ( USD | $ 7.26 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |