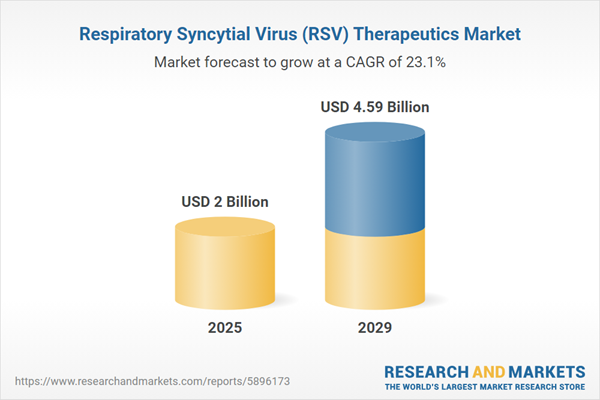
The respiratory syncytial virus (RSV) therapeutics market size is expected to see exponential growth in the next few years. It will grow to $4.59 billion in 2029 at a compound annual growth rate (CAGR) of 23.1%. The growth in the forecast period can be attributed to biologic therapies innovation, emerging antivirals, focus on elderly population, maternal immunization strategies, global research collaborations. Major trends in the forecast period include monoclonal antibodies advancements, nasal vaccines and delivery systems, combination therapies, novel drug delivery systems, home-based treatment options.
The rise in government initiatives and funding is also driving the growth of the respiratory syncytial virus (RSV) therapeutics market in the future. Government and healthcare organization support for the development of RSV therapeutics is motivated by the need to alleviate the significant burden of hospitalizations, severe infections, and healthcare costs associated with RSV, particularly among vulnerable populations such as infants and the elderly. Government programs contribute to the expansion of the RSV therapeutics sector through funding for research, regulatory incentives like fast-track approvals, and public health campaigns that promote awareness and encourage early diagnosis and treatment. For instance, in September 2024, the UK Government launched the world's first national RSV vaccination program to protect both infants and older adults. This program includes vaccines for pregnant women beyond 28 weeks to safeguard newborns, a routine program for those over 75, and a one-off campaign for individuals aged 75 to 79. It aims to alleviate winter pressure on the NHS. Rollouts are set to begin in England, Wales, and Northern Ireland in September, with Scotland starting on August 12. Thus, the increase in government initiatives and funding is propelling the growth of the respiratory syncytial virus (RSV) therapeutics market.
The rising rate of disease is a key factor propelling the growth trajectory of the respiratory syncytial virus (RSV) therapeutics market. A disease signifies a pathological condition affecting a part, organ, or system of an organism due to various causes, including infection, genetic anomalies, or environmental stressors. Given RSV's widespread impact as a common respiratory virus affecting individuals across age groups, the surge in infection rates, particularly among vulnerable demographics such as infants and the elderly, underscores the urgent need for effective therapeutic interventions. For example, according to data released by the Cleveland Clinic in 2023, an estimated 57,000 children under 5 years require hospitalization annually in the U.S. due to RSV. Consequently, the escalating disease rate is a driving force behind the expansion of the respiratory syncytial virus (RSV) therapeutics market.
Developing more effective drugs and vaccines stands as a prominent trend gaining traction within the respiratory syncytial virus (RSV) therapeutics market. Key companies operating in this domain are dedicating their efforts towards creating innovative drugs and vaccines to maintain their market presence. For instance, in May 2023, Pfizer Inc., a renowned pharmaceutical manufacturer in the United States, secured approval from the Food and Drug Administration (FDA), a federal agency responsible for safeguarding public health, for ABRYSVO. This bivalent RSV prefusion F (RSVpreF) vaccine, ABRYSVO (Respiratory Syncytial Virus Vaccine), is administered to prevent lower respiratory tract illnesses caused by RSV in individuals aged 60 and above. ABRYSVO, an unadjuvanted vaccine containing a pair of preF proteins designed to enhance immunity against RSV A and B strains, has demonstrated both safety and efficacy in clinical trials.
Strategic collaborations are emerging as a significant trend gaining momentum within the respiratory syncytial virus (RSV) therapeutics market. Leading companies within this sector are prioritizing the establishment of strategic partnerships to fortify their market positions. For example, in 2023, the Cyrus Poonawalla Group, an India-based biotechnology firm, teamed up with the Serum Institute of India, a prominent biotechnology company specializing in vaccine production, to receive official regulatory clearance for a versatile vaccine. This groundbreaking vaccine aims to combat a wide spectrum of diseases, encompassing polio, tetanus, diphtheria, hepatitis B, mumps, measles, and rubella.
In June 2022, Pfizer Inc., a US-based pharmaceutical manufacturing company, successfully completed the acquisition of ReViral Limited for an undisclosed amount. This strategic move is part of Pfizer's efforts to enhance its pipeline of promising treatment candidates, with a particular focus on sisunatovir. Sisunatovir is an oral antagonist developed to prevent the fusion of the respiratory syncytial virus (RSV) with the host cell. ReViral Limited, headquartered in the UK, is a clinical-stage biotechnology company that specializes in the development of medications targeting RSV.
Major companies operating in the respiratory syncytial virus (RSV) therapeutics market include Johnson & Johnson, F. Hoffmann-La Roche Ltd., Merck & Co. Inc., AbbVie Inc., Novartis AG, Sanofi S.A., AstraZeneca PLC, Abbott Laboratories, GlaxoSmithKline plc., Gilead Sciences Inc., Moderna Inc., Regeneron Pharmaceuticals Inc., Novavax AB, Vir Biotechnology Inc., Lupin Limited, Kyorin Pharmaceutical Co. Ltd., Bavarian Nordic A/S, SciClone Pharmaceuticals Inc., Enanta Pharmaceuticals Inc., Hetero Healthcare Ltd, Inovio Pharmaceuticals Inc., Ascletis Pharma Inc., NanoBio Corporation, Vaxart Inc., Ark Biosciences Private Limited, Pulmocide Ltd.
North America was the largest region in the respiratory syncytial virus (RSV) therapeutics market in 2024. Asia Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the respiratory syncytial virus (rsv) therapeutics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the respiratory syncytial virus (rsv) therapeutics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Respiratory syncytial virus (RSV) therapeutics include various treatments and medications designed to manage and treat infections caused by the respiratory syncytial virus. This virus is known to cause respiratory illness, ranging from mild to severe, with higher risks for infants, young children, older adults, and individuals with weakened immune systems.
The main categories of drugs in respiratory syncytial virus (RSV) therapeutics encompass palivizumab, ribavirin, and others. Palivizumab, a monoclonal antibody developed through DNA recombination, is utilized for respiratory infections in children to prevent complications arising from respiratory syncytial virus infection. Treatments are further classified into immune prophylaxis, supportive care, and antiviral medications, available in various dosage forms such as oral, injectable, and others. These therapeutics are accessible through channels including hospital pharmacies, retail pharmacies, and online pharmacies, catering to patients ranging from pediatrics to adults.
The respiratory syncytial virus (RSV) therapeutics research report is one of a series of new reports that provides respiratory syncytial virus (RSV) therapeutics market statistics, including the respiratory syncytial virus (RSV) therapeutics industry's global market size, regional shares, competitors with a respiratory syncytial virus (RSV) therapeutics market share, detailed respiratory syncytial virus (RSV) therapeutics market segments, market trends and opportunities, and any further data you may need to thrive in the respiratory syncytial virus (RSV) therapeutics industry. This respiratory syncytial virus (RSV) therapeutics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The respiratory syncytial virus (RSV) therapeutics market includes revenues earned by entities by providing services such as home care and respiratory support services. The market value includes the value of related goods sold by the service provider or included within the service offering. The respiratory syncytial virus (RSV) therapeutics market consists of sales of antiviral drugs, monoclonal antibodies, and vaccines. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Respiratory Syncytial Virus (RSV) Therapeutics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on respiratory syncytial virus (rsv) therapeutics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for respiratory syncytial virus (rsv) therapeutics? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The respiratory syncytial virus (rsv) therapeutics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Drug: Palivizumab; Ribavirin; Other Drugs2) By Treatment: Immune Prophylaxis; Supportive Care; Antiviral Medications
3) By Dosage Form: Oral; Injectable; Other Dosage Forms
4) By Patient Type: Pediatrics; Adults
5) By Distribution Channel: Hospital Pharmacies; Retail Pharmacies; Online Pharmacies
Subsegments:
1) By Palivizumab: Monoclonal Antibody2) By Ribavirin: Antiviral Medication
3) By Other Drugs: Cidofovir; Nitazoxanide; Other Experimental or Off-Label Treatments
Key Companies Mentioned: Johnson & Johnson; F. Hoffmann-La Roche Ltd.; Merck & Co. Inc.; AbbVie Inc.; Novartis AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Johnson & Johnson
- F. Hoffmann-La Roche Ltd.
- Merck & Co. Inc.
- AbbVie Inc.
- Novartis AG
- Sanofi S.A.
- AstraZeneca PLC
- Abbott Laboratories
- GlaxoSmithKline plc.
- Gilead Sciences Inc.
- Moderna Inc.
- Regeneron Pharmaceuticals Inc.
- Novavax AB
- Vir Biotechnology Inc.
- Lupin Limited
- Kyorin Pharmaceutical Co. Ltd.
- Bavarian Nordic A/S
- SciClone Pharmaceuticals Inc.
- Enanta Pharmaceuticals Inc.
- Hetero Healthcare Ltd
- Inovio Pharmaceuticals Inc.
- Ascletis Pharma Inc.
- NanoBio Corporation
- Vaxart Inc.
- Ark Biosciences Private Limited
- Pulmocide Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2 Billion |
| Forecasted Market Value ( USD | $ 4.59 Billion |
| Compound Annual Growth Rate | 23.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |