Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Epilepsy, a neurological condition characterized by recurrent seizures, affects a significant global population. Drug-resistant epilepsy - also known as refractory epilepsy - refers to cases in which patients continue to experience seizures despite treatment with two or more appropriate AEDs. This segment represents a substantial unmet clinical need, propelling innovation and investment across the global market landscape.
Key Market Drivers
Increasing Prevalence of Epilepsy
The rising global incidence of epilepsy is a primary factor fueling market growth. According to the World Health Organization (WHO), approximately 50 million individuals worldwide are affected by epilepsy. Of this population, an estimated 30% do not respond adequately to standard anti-seizure therapies, resulting in drug-resistant forms of the disorder.As global demographics shift toward an aging population, the overall prevalence of epilepsy is expected to rise, contributing to an increased burden of drug-resistant cases. This trend underscores the urgent need for advanced therapeutic solutions tailored to this difficult-to-treat patient group.
Additionally, while up to 70% of epilepsy patients could potentially achieve seizure control with appropriate treatment, approximately 75% of individuals in low-income regions lack access to essential medications. This treatment gap highlights the critical importance of early diagnosis, improved access to care, and continued innovation in therapeutic options designed specifically for drug-resistant epilepsy.
Key Market Challenges
Complex and Heterogeneous Disease Profile
One of the most significant challenges in treating drug-resistant epilepsy is the condition’s complex and heterogeneous nature. The disorder comprises a wide range of seizure types, each with distinct etiologies, clinical presentations, and treatment responses. This variability complicates efforts to develop universally effective therapies.The evolving nature of drug-resistant epilepsy further contributes to its clinical complexity. Seizure patterns and drug efficacy may change over time, requiring continuous reassessment and adaptation of treatment strategies. As a result, the development of standardized interventions remains a major hurdle, necessitating a more personalized approach to care.
Key Market Trends
Advancements in Neuroimaging Technologies
Innovations in neuroimaging are significantly enhancing diagnostic and therapeutic capabilities in the drug-resistant epilepsy market. These advanced imaging techniques are transforming clinical approaches by providing deeper insights into brain structure and function.Modern Magnetic Resonance Imaging (MRI) technologies - including functional MRI (fMRI), diffusion tensor imaging (DTI), and magnetic resonance spectroscopy (MRS) - offer high-resolution views of brain activity, connectivity, and subtle structural abnormalities. These tools are instrumental in identifying epileptic foci and guiding surgical or targeted treatment options.
Positron Emission Tomography (PET) also plays a crucial role by detecting metabolic irregularities in brain regions associated with seizure activity. Enhanced by advanced radiotracers and image processing methods, PET imaging facilitates more accurate diagnosis and localization of epileptic zones.
In addition, Single-Photon Emission Computed Tomography (SPECT) is widely used to assess cerebral blood flow during seizures, helping clinicians pinpoint seizure origins and inform personalized treatment planning. Collectively, these advancements are fostering a more precise and effective management paradigm for drug-resistant epilepsy.
Key Market Players
- UCB S.A.
- Jazz Pharmaceuticals
- LivaNova PLC
- NeuroPace, Inc
- Avenue Therapeutics
- Xenon Pharmaceuticals Inc.
- Marinus Pharmaceuticals
- PTC Therapeutics
- Aquestive Therapeutics
- Neuroelectrics
Report Scope:
In this report, the Global Drug-Resistant Epilepsy Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Drug-Resistant Epilepsy Market, By Treatment Type:
- Neuromodulation Therapy
- Antiseizure Medications
- Benzodiazepines
- Resective Epilepsy Surgery
- Specific Metabolic Treatment
- Specific Genetic Treatment
- Immunotherapy
Drug-Resistant Epilepsy Market, By End User:
- Hospitals & Clinics
- Ambulatory Care Centers
- Others
Drug-Resistant Epilepsy Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Drug-Resistant Epilepsy Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- UCB S.A.
- Jazz Pharmaceuticals
- LivaNova PLC
- NeuroPace, Inc
- Avenue Therapeutics
- Xenon Pharmaceuticals Inc.
- Marinus Pharmaceuticals
- PTC Therapeutics
- Aquestive Therapeutics
- Neuroelectrics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
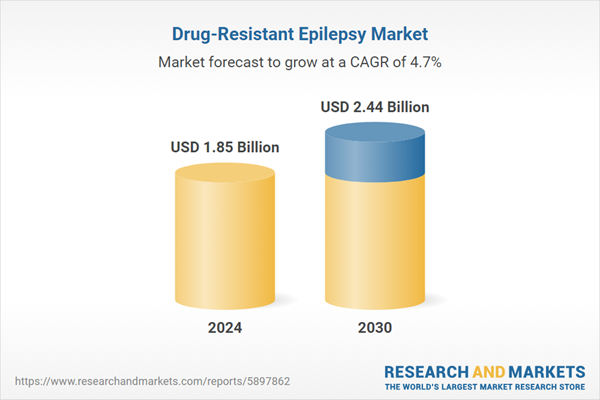
| Estimated Market Value ( USD | $ 1.85 Billion |
| Forecasted Market Value ( USD | $ 2.44 Billion |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |