Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The emergence of precision medicine and personalized therapeutic strategies is further influencing the development of targeted treatments for ATS. Although current treatment options remain limited, ongoing research into potassium channel modulators and gene therapies holds significant promise. Increased investment from pharmaceutical companies and research institutions focused on addressing the unmet needs of ATS patients is also contributing to market growth.
Key Market Drivers
Advancements in Genetic Diagnostics
The growth of the ATS market is strongly supported by progress in genetic diagnostics and electrophysiology, which have significantly enhanced the identification and understanding of this rare autosomal dominant condition. ATS is primarily caused by mutations affecting potassium ion channels, leading to characteristic symptoms including cardiac arrhythmias, muscle weakness, and distinct physical traits. With an estimated prevalence of 0.08 to 0.1 per 100,000 individuals, accurate diagnosis is essential.Improvements in next-generation sequencing (NGS) and genetic screening techniques have enabled earlier and more precise detection, resulting in better disease management. Reports indicate that approximately 76% of physicians are utilizing genetic testing for rare diseases, reflecting a growing emphasis on early identification.
The integration of artificial intelligence (AI) into diagnostic workflows is accelerating the identification of rare mutations associated with ATS, facilitating tailored treatment strategies. Furthermore, the development of rare disease registries and patient databases is enriching the collective understanding of disease progression and treatment efficacy. Regulatory support for orphan drug development and accelerated approval pathways is also fostering innovation in therapy development for ATS.
Key Market Challenges
Diagnostic Delays Due to Complex Symptomatology
A primary challenge in the ATS market lies in diagnostic delays, driven by the condition’s varied and often ambiguous symptom presentation. Patients may exhibit a broad range of clinical manifestations, including cardiac arrhythmias, episodes of muscle paralysis, and unique facial features - symptoms that, while indicative of ATS, are not exclusive to it.This clinical variability frequently leads to misdiagnoses, as symptoms may be mistaken for more common cardiac or neuromuscular conditions. Consequently, patients often receive inappropriate treatments that fail to address the underlying genetic cause.
Furthermore, limited awareness of ATS among both the general public and healthcare professionals exacerbates diagnostic delays. Due to the rarity of the condition, physicians may not consider ATS early in the diagnostic process, resulting in prolonged patient journeys involving multiple specialists and extensive testing before a definitive diagnosis is reached.
These delays can be particularly detrimental in cases involving cardiac complications, where timely intervention is essential to prevent severe outcomes.
Key Market Trends
Cutting-Edge Genetic Diagnostics and Personalized Care
Technological advancements in genetic diagnostics - particularly next-generation sequencing (NGS) and whole exome sequencing (WES) - are revolutionizing the diagnosis and management of ATS. These techniques enable the rapid and accurate identification of pathogenic mutations, including those in the KCNJ2 and CACNA1S genes, which are commonly associated with ATS.As a result, clinicians can now deliver faster, more accurate diagnoses, facilitating timely intervention and access to appropriate treatments. These developments have also deepened scientific understanding of ATS at the molecular level, allowing researchers to explore genotype-phenotype relationships and identify novel therapeutic targets.
This expanding knowledge base is supporting the development of more effective, genetically informed treatments and is accelerating progress toward personalized medicine approaches in the ATS space. Moreover, genetic diagnostics are increasingly being used for carrier screening among family members, enabling early detection, proactive monitoring, and preventative care - further driving demand for ATS-related medical services and diagnostics.
Key Market Players
- Merck KGA.
- Grevis Pharmaceuticals
- Xeris Pharma
- Novartis AG
- Advanz Pharmaceuticals
- Alembic Pharmaceuticals
- Avet Pharmaceuticals
- Hikma Pharmaceuticals
- Micro Labs
- Advagen Pharma
Report Scope:
In this report, the Global Andersen-Tawil Syndrome Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Andersen-Tawil Syndrome Market, By Disease Type:
- Type 1
- Type 2
Andersen-Tawil Syndrome Market, By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Andersen-Tawil Syndrome Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Andersen-Tawil Syndrome Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Merck KGA.
- Grevis Pharmaceuticals
- Xeris Pharma
- Novartis AG
- Advanz Pharmaceuticals
- Alembic Pharmaceuticals
- Avet Pharmaceuticals
- Hikma Pharmaceuticals
- Micro Labs
- Advagen Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
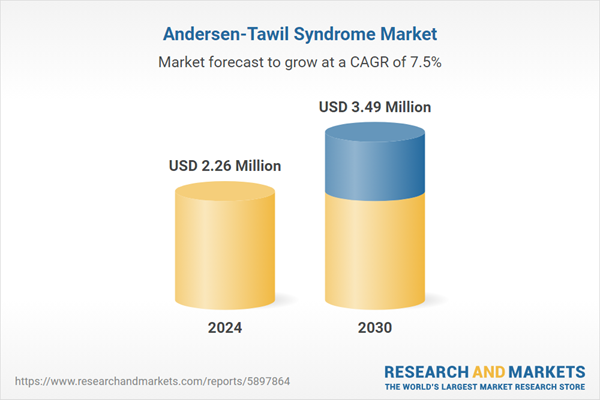
| Estimated Market Value ( USD | $ 2.26 Million |
| Forecasted Market Value ( USD | $ 3.49 Million |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |