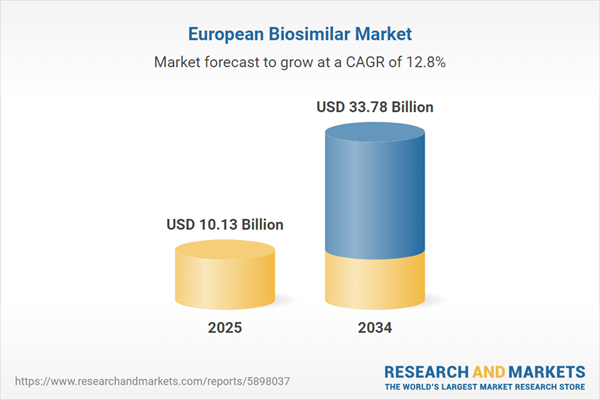
Europe Biosimilar Market Overview
Biosimilars are biologic medical products that are highly similar to an approved reference biologic, with no clinically meaningful differences in terms of safety, efficacy, and quality. These products are made from living organisms and are used to treat various conditions such as cancer, autoimmune diseases, and diabetes. Biosimilars offer a more affordable alternative to expensive biologic therapies, making treatments more accessible to patients. Their development follows rigorous regulatory guidelines to ensure they meet the same standards as their reference products. The growing adoption of biosimilars helps reduce healthcare costs while expanding treatment options for patients.Europe Biosimilar Market Growth Drivers
Rising Incidence of Chronic Diseases
The growing demand for affordable biologics and the increasing healthcare burden due to chronic conditions like psoriasis and inflammatory bowel disease are key drivers of the biosimilar market in Europe. For instance, in July 2024, STADA and Alvotech launched Uzpruvo®, the first approved biosimilar to Stelara® in Europe, across a majority of European countries. This launch follows the expiration of the reference molecule patent, offering patients access to a life-altering treatment for conditions in gastroenterology, dermatology, and rheumatology. As national price approvals are secured, Uzpruvo® is expected to increase competition and reduce healthcare costs in the region, ultimately driving the growth of the biosimilar market. Over the forecast period, the expanding access to high-quality, cost-effective treatments is poised to fuel significant market growth.Europe Biosimilar Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Increasing Adoption of Biosimilars in Oncology Treatments Driving Growth
A key trend in the market is the growing adoption of biosimilars for oncology treatments. With rising cancer incidences and the high cost of biologic therapies, biosimilars provide a more affordable alternative. The European Union’s proactive regulatory framework has facilitated market entry, enabling biosimilar products to offer patients effective and lower-cost treatment options. This trend is expected to fuel market growth in oncology as healthcare systems increasingly prioritise cost-effective solutions. Continued clinical trials and new product approvals will further stimulate the expansion of biosimilars in this high-demand therapeutic area.Application of Biosimilars in Autoimmune Disease Treatment to Boost Europe Biosimilar Market Demand
Another emerging trend is the expansion of biosimilars in autoimmune disease treatment across Europe. Conditions such as rheumatoid arthritis, psoriasis, and Crohn’s disease require long-term biologic therapies, and biosimilars offer a cost-effective option without compromising treatment efficacy. With increasing patient numbers and pressure on healthcare budgets, biosimilars provide a viable alternative to traditional biologics. As the regulatory landscape evolves to support biosimilar uptake and new formulations are developed, the segment is poised for significant growth. Biosimilars’ potential in autoimmune diseases is driving increasing investments and expanding market opportunities across the region.Increased Regulatory Support and Approvals Enhancing Europe Biosimilar Market Value
Regulatory support and approvals are crucial drivers for the market. The European Medicines Agency (EMA) has established a strong regulatory framework to ensure the safety and efficacy of biosimilars. This trend has accelerated market development by simplifying the approval process, thus enhancing the availability of biosimilars in the market. The EMA's focus on reducing barriers for biosimilars and providing clear guidelines has instilled confidence in both healthcare providers and patients. As a result, more biosimilars are expected to enter the market, boosting competition and expanding treatment options for chronic conditions.Rising Patient Awareness Strengthening Europe Biosimilar Market Growth
An essential market trend in the market is the rising patient awareness surrounding biosimilars. Patients are becoming more informed about biosimilar options due to increased education from healthcare providers and patient advocacy groups. This awareness is helping to overcome misconceptions about the safety and efficacy of biosimilars compared to their reference biologics. As more patients opt for biosimilars, healthcare systems experience cost savings, improving market value. This trend will continue to drive market expansion as public confidence in biosimilars grows, alongside the rising demand for affordable alternatives in various therapeutic areas.Europe Biosimilar Market Segmentation
Europe Biosimilar Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Molecule
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Others
Market Breakup by Manufacturing Type
- In-house Manufacturing
- Contract Manufacturing
Market Breakup by Indication
- Auto-Immune Diseases
- Blood Disorders
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
- Others
Market Breakup by Country
- United Kingdom
- Germany
- France
- Italy
- Others
Europe Biosimilar Market Share
Infliximab to Lead the Market Share by Molecule
Infliximab holds the largest market share due to its extensive use in treating auto-immune conditions such as rheumatoid arthritis and Crohn’s disease. Its affordability compared to branded biologics has driven widespread adoption, supported by growing regulatory approvals. The increasing prevalence of auto-immune diseases and ongoing cost-containment measures in healthcare systems further boost its market value. Infliximab is expected to remain dominant during the forecast period, benefiting from rising awareness about biosimilars and the introduction of more advanced formulations that enhance treatment efficacy and patient compliance.Europe Biosimilar Market Segmentation by Manufacturing Type to Witness Significant Growth
In-house manufacturing dominates the market share by manufacturing type as it allows manufacturers to maintain stringent control over production quality, scalability, and costs. Leading biosimilar companies heavily invest in proprietary manufacturing facilities to meet rising demand efficiently. The growing emphasis on ensuring uninterrupted supply chains, compliance with European regulatory standards, and technological advancements in bioprocessing bolster this segment's growth. During the forecast period, in-house manufacturing is expected to drive the market by enabling innovation and reducing dependency on external contractors, thereby ensuring better cost-effectiveness and reliability in biosimilar production.Auto-Immune Diseases to Hold a Major Share of the Market
The auto-immune diseases segment leads the Europe biosimilar market segment on the basis of indication, driven by the high incidence of conditions like rheumatoid arthritis, psoriasis, and ulcerative colitis. Biosimilars such as infliximab and etanercept have gained significant traction in this therapeutic area, offering cost-effective alternatives to originator biologics. Increased healthcare expenditure and greater patient access to biosimilars, aided by favourable government policies, propel this segment’s dominance. Looking ahead, the rising adoption of biosimilars for treating chronic auto-immune diseases is expected to sustain growth, supported by advancements in therapeutic formulations and patient-focused healthcare solutions.Europe Biosimilar Market Analysis by Region
Germany will likely hold the largest market share in the European market due to its advanced healthcare infrastructure, supportive regulatory environment, and strong demand for cost-effective treatments. The country has made significant investments in biosimilar adoption, particularly through its healthcare reimbursement system, which incentivises the use of biosimilars. Germany's well-established pharmaceutical sector and the presence of leading biotech companies further contribute to its market leadership. Additionally, Germany's central role in the European Union's regulatory processes allows for quicker market access for biosimilars. As the largest economy in Europe, Germany is poised to continue driving the market with its emphasis on high-quality, affordable biologic therapies.Leading Players in the Europe Biosimilar Market
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, clinical trials analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Pfizer, Inc
Headquartered in New York, USA, Pfizer, Inc. was established in 1849. A global leader in pharmaceuticals, it offers a diverse portfolio including biosimilars, vaccines, oncology treatments, and more. In the European biosimilar market, Pfizer focuses on developing high-quality biosimilars to address conditions like rheumatoid arthritis, oncology, and diabetes. Its prominent biosimilars include infliximab (Inflectra) and somatropin (Ruxience). With a commitment to increasing access to affordable medicines, Pfizer plays a significant role in the expansion and development of biosimilar therapies in Europe.Celltrion Inc
Founded in 2002 and headquartered in Incheon, South Korea, Celltrion Inc. is a major player in the global biosimilars market. Known for pioneering the development of monoclonal antibody biosimilars, its portfolio in Europe includes biosimilars for autoimmune diseases and oncology, such as infliximab (Remsima) and trastuzumab (Herzuma). Celltrion has built a strong presence in Europe, focusing on making high-quality biologics more accessible. Its advanced manufacturing technologies and extensive regulatory expertise support its growth in the European biosimilar market, enhancing the affordability and availability of key biologic treatments.Novartis AG
Established in 1996 and headquartered in Basel, Switzerland, Novartis AG is a global healthcare leader with a broad portfolio spanning pharmaceuticals, generics, and biosimilars. In Europe, its biosimilars division, led by its Sandoz division, is a key player in the biosimilar market. Novartis’ biosimilars include treatments for oncology, immunology, and ophthalmology, such as rituximab (Rixathon) and pegfilgrastim (Ziextenzo). With a focus on expanding access to critical biologics, Novartis remains committed to increasing the availability of biosimilars, contributing to the overall market growth in Europe.Amgen Inc
Founded in 1980 and headquartered in Thousand Oaks, California, USA, Amgen is a global biotechnology company renowned for its innovative biologics and biosimilars. In Europe, Amgen focuses on expanding its biosimilars portfolio, including products for oncology and rheumatoid arthritis. Key biosimilars include infliximab (Amgevita) and etanercept (Benepali). With a commitment to high-quality biologic medicines, Amgen continues to lead the European biosimilars market, providing affordable alternatives to patients and ensuring greater access to effective treatments across various therapeutic areas.Other key players in the market include Eli Lilly and Company, Samsung Bioepis, Sanofi SA, Dr. Reddy’s Laboratories Ltd, and Boehringer Ingelheim.
Key Questions Answered in the Europe Biosimilar Market
- What was the Europe biosimilar market value in 2024?
- What is the Europe biosimilar market forecast outlook for 2025-2034?
- What is market segmentation based on molecule?
- How is the market segmented based on manufacturing type?
- How is the market segmented based on indication?
- What are the major factors aiding the Europe biosimilar market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major Europe biosimilar market trends?
- Which molecule method will lead the market segment?
- Which manufacturing type will lead the market segment?
- Which indication will lead the market segment?
- Who are the key players involved in the Europe biosimilar market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer, Inc.
- Celltrion Inc.
- Novartis AG
- Amgen Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 300 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 10.13 Billion |
| Forecasted Market Value ( USD | $ 33.78 Billion |
| Compound Annual Growth Rate | 12.8% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 4 |