Evolving Applications of Lateral Flow Assays Fuel South & Central America Lateral Flow Assay Market
Lateral flow assays are widely used for infectious disease diagnosis, heart disease diagnosis, and veterinary applications. However, the scope of their applications has expanded in recent years. Behavioral health, saliva-based diagnostics, biological warfare (detection of Bacillus anthracis), environmental testing (detection of contaminating enzymes in production facilities), agriculture (detection of genetically modified organisms and quality control of plants), food microbiology (Detection of E. coli O157, Salmonella, Listeria, and other food spoilage bacteria) have emerged as new application areas for lateral flow assays.Atlas-Link Biotech (Beijing) provides FASPIT Antigen Saliva Test Kit. NOVA Test SARS-CoV-2 Antigen Saliva Test Kit (Colloidal Gold Immunochromatography) is a lateral flow assay that uses the double-antibody sandwich method to detect the SARS-CoV-2 nucleocapsid protein from saliva specimens from patients who are suspected of SARS-CoV-2 by a healthcare provider. Only certified laboratories that meet the requirements for performing moderate, high, or waived complexity tests can perform such saliva-based tests. The SARS-CoV-2 antigen saliva test kits provide preliminary test results, with negative results that don't preclude SARS-CoV-2 infection.
South & Central America Lateral Flow Assay Market Overview
The South & Central America lateral flow assay market is segmented into Brazil, Argentina, and the Rest of South & Central America. The increase in the adoption of lateral flow assay-based rapid antigen test kits, a rise in the incidence of infectious diseases, and increasing market developments are among the factors contributing to the market growth in the region.Brazil has a well-established medical device industry. The country has the region's most significant medical industry and is the US's largest importer of medical products.
The International Trade Administration (the US Department of Commerce) reported that in 2020, Brazil increased imports of medical devices by 12.9%, reaching US$ 6.2 billion in imports. The Brazilian National Health Surveillance Agency (ANVISA) regulates the safe usage of medical devices and in vitro diagnostic (IVD) products. In January 2022, ANVISA approved a new regulation allowing pharmacies and healthcare retailers to facilitate the distribution and sale of at-home self-test kits to detect SARS-CoV-2. ANVISA also notified Brazilian Registration Holders (BRH) to register any COVID-19 rapid antigen self-test device before importing, marketing, or distributing across the nation. Thus, the approval of regulation to distribute and sell COVID-19 rapid test kits in Brazil fueled the market growth.
South & Central America Lateral Flow Assay Market Segmentation
The South & Central America lateral flow assay market is categorized into product type, technique, test type, application, end user, and country.Based on product type, the South & Central America lateral flow assay market is bifurcated into kits & reagents and lateral flow readers. The kits & reagents segment held a larger South & Central America lateral flow assay market share in 2022.
In terms of technique, the South & Central America lateral flow assay market is segmented into sandwich assay, competitive assays, and multiplex detection assay. The sandwich assay segment held the largest South & Central America lateral flow assay market share in 2022.
By test type, the South & Central America lateral flow assay market is divided into lateral flow immunoassay and nucleic acid lateral flow assay. The lateral flow immunoassay segment held a larger South & Central America lateral flow assay market share in 2022.
Based on application, the South & Central America lateral flow assay market is categorized into clinical testing, veterinary diagnostics, food safety & environment testing, and drug development & quality testing. The clinical testing segment held the largest South & Central America lateral flow assay market share in 2022.
By end user, the South & Central America lateral flow assay market is segmented into hospitals and clinics, diagnostics laboratories, homecare, veterinary clinics, pharmaceutical & biotechnology companies, and others. The hospitals and clinics segment held the largest South & Central America lateral flow assay market share in 2022.
By country, the South & Central America lateral flow assay market is segmented into Brazil, Argentina, and the Rest of South & Central America. Brazil dominated the South & Central America lateral flow assay market share in 2022.
F. Hoffmann-La Roche Ltd, Siemens Healthineers AG, Becton Dickinson and Co, PerkinElmer Inc, Hologic Inc, QIAGEN NV, bioMerieux SA, QuidelOrtho Corp, Abbott Laboratories, Merck KGaA, Bio-Rad Laboratories Inc, and Thermo Fisher Scientific Inc are some of the leading companies operating in the South & Central America lateral flow assay market.
Table of Contents
Companies Mentioned
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers AG
- Becton Dickinson and Co
- PerkinElmer Inc
- Hologic Inc
- QIAGEN NV
- bioMerieux SA
- QuidelOrtho Corp
- Abbott Laboratories
- Merck KGaA
- Bio-Rad Laboratories Inc
- Thermo Fisher Scientific Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 128 |
| Published | May 2024 |
| Forecast Period | 2022 - 2030 |
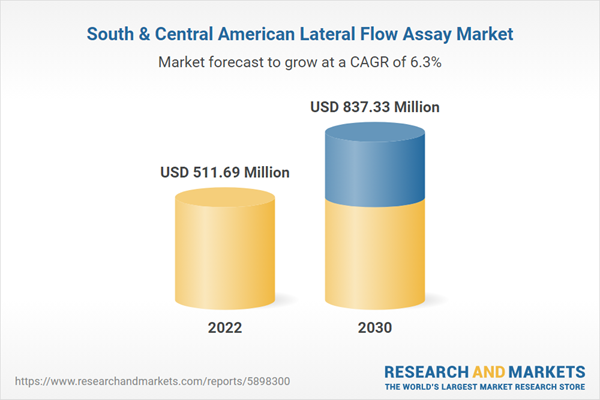
| Estimated Market Value ( USD | $ 511.69 Million |
| Forecasted Market Value ( USD | $ 837.33 Million |
| Compound Annual Growth Rate | 6.3% |
| No. of Companies Mentioned | 12 |