Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
This buildup of Gb3 primarily affects the cells of blood vessels, kidneys, heart, and nervous system, leading to a wide range of symptoms and complications. Symptoms of Fabry disease often appear in childhood or adolescence, although the age of symptom onset and their severity can vary widely among affected individuals. One of the hallmark symptoms of Fabry disease is neuropathic pain, which can be severe and chronic.
This pain typically affects the extremities, such as the hands and feet, and is often described as burning or tingling. For instance, according to the National Fabry Disease Foundation (NFDF) designates April as Fabry Disease Awareness Month each year in the United States. Awareness programs focus on educating the public, patients, caregivers, and healthcare professionals. These initiatives aim to improve the understanding, diagnosis, and management of Fabry disease, ultimately contributing to market growth throughout the study period.
Key Market Drivers
Advancements in Diagnosis
Genetic testing has become a cornerstone in the diagnosis of Fabry disease. Advances in genetic sequencing technology have made it more accessible and cost-effective to identify specific mutations in the GLA gene, which is responsible for the disease. Genetic testing can confirm the presence of Fabry disease and provide information about the specific genetic mutations involved. In some regions, newborn screening programs have been implemented to identify Fabry disease in infants shortly after birth. This early detection allows for prompt intervention and treatment, potentially preventing or delaying the onset of symptoms and organ damage. Researchers have been investigating biomarkers associated with Fabry disease.Biomarkers are measurable substances in the body that can indicate the presence of a disease or its progression. Biomarker research can aid in early diagnosis and monitoring the effectiveness of treatment. Clinical diagnostic criteria for Fabry disease have been refined and standardized. Healthcare professionals now have clearer guidelines and criteria to assist in diagnosing disease based on clinical symptoms and genetic testing results. For instance, in December 2022, Bio Sidus SA sponsored a clinical trial to assess the efficacy and safety of AGA BETA BS in FD patients previously stabilized with Fabrazyme. Also, Fabry disease pipeline products continue to drive market growth. For example, in August 2021, the European Commission approved Amicus Therapeutics' Galafold for long-term treatment of Fabry disease in patients aged 12 and above with an amenable mutation.
Key Market Challenges
Limited Patient Population
The small patient population with Fabry disease results in a limited market size for pharmaceutical companies. This can make it less economically attractive for these companies to invest in research and development of treatments for disease. Developing and gaining regulatory approval for new treatments, including clinical trials and research, is costly. In the case of rare diseases like Fabry disease, the small number of potential patients can make it challenging to recoup these expenses. Due to the small market size, the revenue potential for treatments for Fabry disease may be limited compared to drugs for more common conditions. This can affect the profitability of drug development efforts.Limited patient populations can result in challenges related to patient access to treatments and affordability. Even if effective treatments exist, they may not be accessible to all patients due to cost or availability. The rarity of Fabry disease can lead to delayed diagnosis or misdiagnosis, as healthcare providers may not be familiar with the condition.
This can result in patients not receiving appropriate treatment until the disease has progressed. Limited patient populations can also result in a scarcity of clinical data on the disease and its treatment. This can make it challenging for healthcare providers to make informed treatment decisions. Recognizing the challenges posed by rare diseases, some regulatory agencies provide incentives for orphan drug development. These incentives can include extended market exclusivity and reduced regulatory fees.
Key Market Trends
Biomarker Development
Biomarkers can aid in the early diagnosis of Fabry disease, allowing for timely intervention and treatment initiation. Early diagnosis is essential for preventing or delaying the onset of symptoms and organ damage. Biomarkers can be used to monitor disease progression and assess the effectiveness of treatment over time. This enables healthcare providers to tailor treatment plans to individual patients' needs. Certain biomarkers may be associated with the severity of Fabry disease. Identifying these biomarkers can help predict disease outcomes and guide treatment decisions. Biomarkers can help determine how well a patient is responding to treatment. If treatment is effective, biomarkers may show improvements in disease-related indicators.Biomarkers can serve as valuable endpoints in clinical trials of new Fabry disease treatments. They provide objective measures of treatment efficacy and safety. The development of biomarkers supports the concept of personalized medicine, where treatments are tailored to individual patients based on their biomarker profiles. This approach can optimize treatment outcomes. Biomarkers provide insights into the underlying molecular and genetic mechanisms of Fabry disease.
This knowledge can inform drug development efforts, leading to more targeted and effective therapies. Biomarkers can be used to stratify Fabry disease patients into different subgroups based on disease characteristics. This stratification can guide treatment decisions and improve patient outcomes. Biomarkers can help reduce diagnostic delays by providing objective evidence of Fabry disease. This is particularly important because Fabry disease is often underdiagnosed or misdiagnosed.
Key Market Players
- Sanofi (Genzyme Corporation)
- Takeda Pharmaceutical Company Limited
- Amicus Therapeutics, Inc
- ISU ABXIS Co Ltd.
- JCR Pharmaceuticals Co., Ltd.
- Protalix BioTherapeutics Inc.
- Chiesi Farmaceutici S.p.A.
- Freeline Therapeutics Holdings PLC
- Yuhan Corporation
- M6P Therapeutics Inc.
Report Scope:
In this report, the Global Fabry Disease Treatment Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Fabry Disease Treatment Market, By Drugs:
- Agalsidase Beta
- Migalastat
- others
Fabry Disease Treatment Market, By Treatment:
- Enzyme Replacement Therapy (ERT)
- Chaperone Treatment
- Substrate Reduction Therapy (SRT)
- Others
Fabry Disease Treatment Market, By Route of Administration:
- Oral
- Parenteral
- Others
Fabry Disease Treatment Market, By Distribution Channel:
- Hospital pharmacies
- Retail pharmacies
- Online Pharmacies
Fabry Disease Treatment Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Fabry Disease Treatment Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Sanofi (Genzyme Corporation)
- Takeda Pharmaceutical Company Limited
- Amicus Therapeutics, Inc
- ISU ABXIS Co Ltd.
- JCR Pharmaceuticals Co., Ltd.
- Protalix BioTherapeutics Inc.
- Chiesi Farmaceutici S.p.A.
- Freeline Therapeutics Holdings PLC
- Yuhan Corporation
- M6P Therapeutics Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
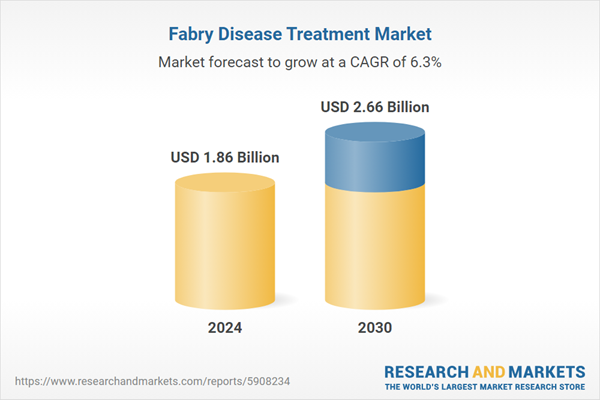
| Estimated Market Value ( USD | $ 1.86 Billion |
| Forecasted Market Value ( USD | $ 2.66 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |