Therapeutic Drug Monitoring: Introduction
Therapeutic drug monitoring is the periodic measurement of several drugs in the bloodstream. It is an evaluation to ensure that a patient is adhering to the prescribed dosage. It is also used to gauge the effectiveness of the medications.Therapeutic drug monitoring is used to optimize the dosages for people who are taking certain medicines, which can be hard to dose due to potential side effects. Such medicines may range from antibiotics, heart drugs, anti-seizure drugs, and drugs to treat autoimmune diseases. It is also known as medicine levels blood test or therapeutic drug levels test.
Global Therapeutic Drug Monitoring Market Analysis
Therapeutic drug monitoring plays a critical role in clinical practice. Monitoring drug levels in individual patients allows for patient-specific dose optimisation. Therefore, there has been a notable upswing in the therapeutic drug monitoring market demand, particularly with precision medicine gaining prominence in today's healthcare system.Conventional therapeutic drug monitoring techniques are time-consuming and labour-intensive. They require large sample volumes, along with a substantial number of organic solvents. In some cases, samples must be sent to central laboratories for analysis as well, potentially causing a delay in getting the required results. In this regard, Raman spectroscopy has emerged as a promising technique that offers label-free and non-invasive characteristics that also facilitate smooth, rapid, and precise analysis.
Furthermore, UCL researchers have also developed a new drug-monitoring tool called ChromaDose that calculates the efficacy of cancer medicine in a patient's bloodstream. It is aimed at reducing heart issues and other side effects experienced by kids receiving chemotherapy. This point-of-care tool can be made available in hospitals by 2025. In addition, there has been ongoing research on dual-colour bioluminescent sensor proteins for therapeutic drug monitoring of antitumor antibodies. As research and development continue to progress, therapeutic drug monitoring market value will increase in the forecast period.
Global Therapeutic Drug Monitoring Market Segmentation
Global Therapeutic Drug Monitoring Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Consumables
- Devices
- Immunoassay Analyzers
- Chromatography and MS Detectors
- Clinical Chemistry Analyzers
- Others
Market Market Breakup by Type
- Immunoassays
- Proteomic Technologies
Market Breakup by Drug Class
- Antiepileptic
- Antiarrhythmic
- Immunosuppressants
- Antibiotic Drugs
- Bronchodilator Drugs
- Psychoactive Drugs
- Others
Market Market Breakup by End User
- Hospitals
- Commercial and Private Laboratories
- Others
Market Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Therapeutic Drug Monitoring Market Overview
With the prominence of precision medicine, researchers have turned towards therapeutic drug monitoring for treating diseases where accurate measurement of drugs plays a vital role in the treatment. For instance, in diseases like inflammatory bowel disease and paediatric cystic fibrosis treatment, therapeutic drug monitoring is being used to enhance treatment outcomes. This indicates the certainty of therapeutic drug monitoring market growth in upcoming years.Considering the growing population, developing infrastructure, and increased technical advancements in the Asia Pacific region, the market for therapeutic drug monitoring is expected to observe a steep growth. Increased awareness regarding diagnostics is another factor fuelling the growth, in contrast to the historical period, when the United States led the therapeutic drug monitoring market share. This can be attributed to the presence of major healthcare giants in the area, committed to work on new innovations, envisioned at improving patient treatment and overall experience.
Global Therapeutic Drug Monitoring Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Abbott
- Thermo Fisher Scientific
- F. Hoffmann-La Roche
- Siemens Healthineers AG
- Danaher Corporation
- Bio-Rad Laboratories
- Biomérieux SA
- Theradiag
- Grifols S.A.
- Exagen Inc.
- Ark Diagnostics, Inc.
- R-Biopharm A
- Apdia Group
- Biotez Berlin Buch GmbH
- Eagle Biosciences, Inc.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- ABBOTT
- THERMO FISHER SCIENTIFIC
- F. HOFFMANN-LA ROCHE
- SIEMENS HEALTHINEERS AG
- DANAHER CORPORATION
- BIO-RAD LABORATORIES
- BIOMÉRIEUX SA
- THERADIAG
- GRIFOLS S.A.
- EXAGEN INC.
- ARK DIAGNOSTICS, INC.
- R-BIOPHARM A
- APDIA GROUP
- BIOTEZ BERLIN BUCH GMBH
- EAGLE BIOSCIENCES, INC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
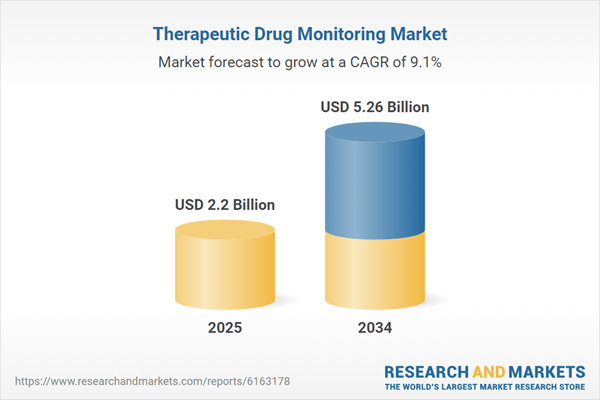
| Estimated Market Value ( USD | $ 2.2 Billion |
| Forecasted Market Value ( USD | $ 5.26 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |